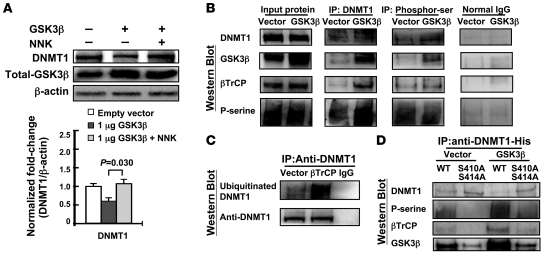
Figure 4. GSK3β and βTrCP interact with DNMT1 protein and enhance DNMT1 degradation.
(A) A549 cells were transfected with pCMV-SPORT6-GSK3β, then treated with or without NNK. The GSK3β pathway promoted DNMT1 protein degradation, which was attenuated by NNK treatment. Data are mean ± SEM (n = 3). (B) Cell lysates were immunoprecipitated with anti-DNMT1 or anti–phospho-Ser antibody and then Western blotted. DNMT1 protein formed complexes with GSK3β and βTrCP proteins. Increase DNMT1 phosphorylation was mediated by exogenous GSK3β, which increased the interaction between DNMT1 and βTrCP. Normal IgG was used as a negative control. (C) βTrCP increased the ubiquitination level of DNMT1. A549 cells were transfected with pCMV-SPORT6-βTrCP for 24 hours, after which cell lysates were immunoprecipitated with anti-DNMT1 and then western blotted. (D) Site-directed mutagenesis of both Ser410 (S410A) and Ser414 (S414A) on DNMT1 protein decreased the phosphorylation level of DNMT1 protein by GSK3β and disrupted the interaction between βTrCP and DNMT1. Cells were transfected with WT or mutant His-tag DNMT1 expression vector and exogenous GSK3β or vector control. Cell lysates were immunoprecipitated with anti–His-tag antibody and then Western blotted.