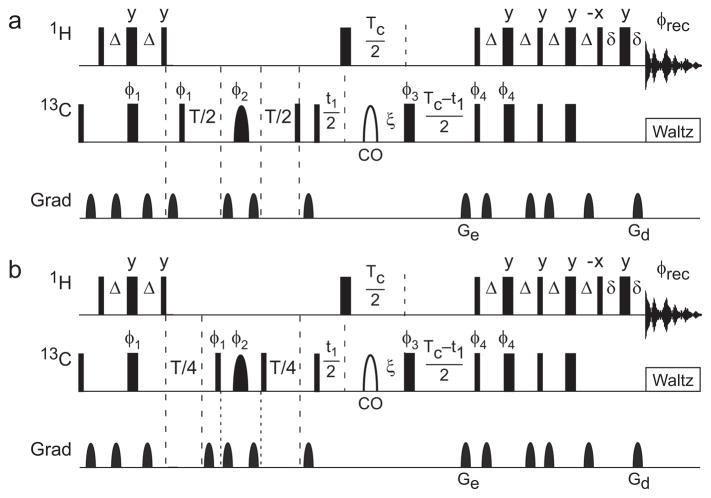
Figure 2.
Hahn spin echo pulse sequence for fractionally 13Cα labeled proteins. Narrow and wide solid rectangular bars represent 90° and 180° pulses, respectively. The shaped pulse represented by a solid semi-ellipse is a Reburp 180° 13Cα refocusing pulse, and the shaped pulse represented by a open semi-ellipse is an off-resonance 4% truncated Gaussian pulse applied at the carbonyl 13C frequency. The power of the rectangular 180° pulse during the constant time period is adjusted to have a null at the carbonyl 13C frequency. Delays are defined as follows: Δ = 1/(4JCH) = 1.75 ms, ξ = 1/(4JCC′) = 4.54 ms, T is the relaxation delay, TC = 1/2JCC = 14.28 ms is the constant time period, t1 is the indirect carbon-frequency labeling delay, and δ is long enough to encompass the final gradient pulse. Pulse phases are x unless otherwise illustrated. Phase cycles are φ1 = x, −x; φ2 = 8(y), 8(−x). 8(−y), 8(x); φ3 = 2(x), (2y), 2(−x), 2(−y); φ4 = x; φrec = 2(x, −x, −x, x), 2(−x, x, x, −x). Unlabeled gradients are employed to suppress unwanted coherences and artifacts while Ge and Gd are encoding and decoding gradients, respectively, for echo/anti-echo coherence selection; selection is obtained by inverting the signs of φ4 and Ge (Palmer, et al., 1991, Kay, et al., 1992).