Abstract
Object
Investigation of the effect of lymphatic inhibition on joint and draining lymph node pathology during the course of arthritis progression in mice.
Method
TNF transgenic (TNF-Tg) mice were used as a model of chronic inflammatory arthritis. Mice received contrast enhanced MRI to obtain ankle and knee joint synovial volumes and draining popliteal lymph node (PLN) volumes before and 8 weeks after treatment with VEGFR-3 or VEGFR-2 neutralizing antibodies, or isotype IgG. The animals were subjected to near-infrared lymphatic imaging to determine the effect of VEGFR-3 neutralization on lymph transport from paws to draining PLNs prior to sacrifice. Lymphatic vessel formation and morphology of joints and PLNs were examined by histology, immunohistochemistry, and RT-PCR.
Results
Compared to IgG treatment, VEGFR-3 neutralizing antibody treatment significantly decreased the size of PLNs, the number of lymphatic vessels in joints and PLNs, the lymphatic drainage from paws to PLNs, and the number of VEGF-C expressing CD11b+ myeloid cells in PLNs. However, it increased the synovial volumes and inflammatory area in ankle and knee joints. VEGFR-2 neutralizing antibody, in contrast, inhibited both lymphangiogenesis and joint inflammation.
Conclusion
Lymphangiogenesis and lymphatic drainage are reciprocally related to the severity of joint lesions during the development of chronic arthritis. Lymphatic drainage plays a beneficial role in controlling the progression of chronic inflammation.
Keywords: Lymphatic drainage, lymphangiogenesis, inflammation, lymph nodes, in vivo imagining
Lymphatic vessels are present in almost all tissues of the body. They are composed of an extensive network of thin-walled capillaries that drain protein-rich lymph from extracellular spaces (1). Under normal conditions, the major functions of the lymphatic system include maintenance of tissue fluid homeostasis, absorption of fatty acids, and mediation of the afferent immune response (2, 3). Recent studies show increasing evidence that the lymphatic system also plays key roles in disease processes such as cancer metastasis, lymphedema, obesity, and inflammation (4, 5).
Lymphatic endothelial cell proliferation and lymphatic hyperplasia are reported in psoriatic skin lesions in humans and in chronic skin inflammation in mice (6). Kidney transplant rejection is frequently accompanied by enhanced lymphangiogenesis and production of lymphatic endothelial cell-derived chemokines in grafted tissues (7). Synovial specimens from patients with rheumatoid arthritis (RA) and osteoarthritis have an increased number of lymphatic vessels and increased expression of the lymphatic growth factor, vascular endothelial growth factor-C (VEGF-C) (8, 9). Furthermore, clinical reports have described larger lymph nodes (10) and increased lymphatic flow rates in lymphatic vessels draining arthritic joints in RA patients (11). Similarly, recent studies in animal models of RA demonstrated increased lymphatic vessel formation in inflamed joints and in draining popliteal lymph nodes (PLN) (12–14). These clinical and preclinical studies have demonstrated that inflammation stimulates proximal lymphangiogenesis in the joints and distal lymphangiogenesis in the draining lymph nodes. Thus, inflammation is the primary cause of lymphangiogenesis in arthritis. However, the effects of inflammation-induced lymphangiogenesis on joint inflammation in RA have yet to be determined.
VEGF-A- and VEGF-C-mediated signaling pathways are centrally involved in inflammatory lymphangiogenesis. VEGF-A signals through VEGF receptors (VEGFR)-1 and VEGFR-2. The effect of VEGF-A on lymphatics is mediated by VEGFR-2. Blockade of VEGF-A/VEGFR-2 interaction, using VEGFR-2 neutralizing antibody, reduces adjuvant-induced (15) and delayed-type hypersensitivity reaction-induced (16) lymphangiogenesis in lymph nodes. VEGF-C signals primarily through VEGFR-3 on lymphatic endothelial cells. VEGFR-3 blockade specifically inhibits the effects of VEGF-C, but not VEGF-A, on lymphatics. VEGFR-3 neutralizing antibody reduces bacterial infection associated-airway inflammation (17) and surgical blockade-induced lymphangiogenesis (18, 19). These studies have established a direct role for VEGF-C:VEGFR3 signaling in inflammation-induced lymphangiogenesis. However, most published studies have used acute inflammation models in which inflammation is triggered within hours to days. The effects of lymphangiogenesis on the natural course of chronic inflammation, such as that occurring in RA, have not been addressed. Specifically, it is not known how newly-generated lymphatic vessels affect drainage from inflamed joints, or if reduced lymphatics exacerbates inflammation. These questions are even more important in chronic inflammation where monocytes/macrophages are the major infiltrating cell type, given the fact that macrophages are the main source of VEGF-C in response to pro-inflammatory cytokines TNF and IL-1 (12, 20, 21)
In the current study, we used TNF transgenic (Tg) mice as a model of chronic inflammatory arthritis (22), and examined the effect of lymphatic inhibition by VEGFR-2 and VEGFR-3 neutralizing antibodies on lymphatic drainage and the severity of joint inflammation. Contrast enhancement (CE) MRI (13, 23) and indocyanine green-near infrared (ICG-NIR) in vivo imaging technologies were used to monitor the changes of synovial and PLN volumes during the 8-week treatment period. We found that VEGFR-3 neutralizing antibody significantly decreased joint and PLN lymphangiogenesis, lymphatic drainage from inflamed paws to PLNs, and the number of VEGF-C expressing CD11b+ myeloid cells in the PLNs. However, it significantly exacerbated joint inflammation. In contrast, VEGFR-2 neutralization inhibited both joint inflammation and lymphangiogenesis. These data indicate that inflammation-induced lymphangiogenesis is an important compensatory mechanism to limit joint inflammation during the course of chronic arthritis, and that improving lymphatic drainage represents a new potential therapeutic strategy for chronic inflammatory disorders.
MATERIAL AND METHODS
Animal experiments
TNF-Tg mice (Tg3647) were originally obtained from Dr. G. Kollias, and were bred with C57BL/6 mice for 8 generations. TNF-Tg mice (2.5-months-old) were treated with anti-mouse VEGFR-2 (DC101), anti-mouse VEGFR-3 (mF4-31C1) neutralizing antibody (ImClone, New York, NY) or rat IgG (Innovative, Southfield, MI) at a dose of 0.8 mg/mouse, twice a week, by intra-peritoneal injection, for 8 weeks. DC101 is a rat monoclonal antibody which specifically blocks the binding of VEGF-A to VEGFR-2 and inhibits tumor growth in mice through an anti-angiogenic mechanism (24). mF4-31C1 is a rat monoclonal antibody which specifically antagonizes the binding of VEGF-C to VEGFR-3 and potently blocks both VEGF-C-enhanced physiological and tumor-induced lymphangiogenesis in a murine tail skin lymphatic generation model (25). The rationale for choosing 2.5 month-old TNF-Tg mice for 8-week treatment is based on our previous experience using antibody therapy in this model. We have shown that 8-week anti-RANK or anti-TNF treatment of 2.5-month-old TNF-Tg mice significantly reduces joint lesions (22, 26).
FITC-dextran footpad injection and PLN confocal microscopy
TNF-Tg mice (4–6-months-old) and wild type (WT) littermates were injected with 10 μl (10 mg/ml) of FITC-dextran (molecular weight, 2,000Kd, Sigma) into the footpad intra-dermally. This size of dextran is too large to enter the blood stream and is routinely used in lymphatic studies (27, 28). The entire PLNs were scanned one hour later under a Confocal microscope. A total of 100 slices about 600~800 μm deep were taken for each node, and 4 nodes from TNF-Tg mice or WT littermates were examined.
CE-MRI
CE-MRI was performed before and after the antibody treatment as previously described (13, 23). Mice were positioned with a hind leg in a newly-developed custom-designed murine dual RF receiver coil (one coil enclosing the ankle joint and another for the knee joint and PLN). Mice were scanned in a Siemens 3 Tesla clinical magnet (Siemens AG, Munich, Germany) as we previously described (13, 23). Analysis was performed with Amira 3.1 (Mercury Computer Systems, Chelmsford, MA). An image registration and subtraction algorithm was used on the pre- and post-contrast images in Amira in order to generate an image of the voxels of contrast enhancement. From this image, a three-dimensional region of interest of muscle tissue was used as a measure of delivered contrast agent, and a threshold of enhancing synovial tissue was generated from this value. Lymph nodes were traced manually and thresholded to define the margin between the node and surrounding fat. Tissue volumes were reconstructed using a surface generation module in Amira.
ICG-NIR lymphatic imaging
To examine the status of lymphatic draining function in the legs of TNF-Tg mice, we established a protocol using ICG-NIR imaging technology according to published literature (29, 30) and our past experience with large animals and human subjects (detailed methods will be published separately). In brief, on the first day, ICG solution (1 μg in 10 μl) was injected into the footpads intra-dermally. Five minutes later, the dynamics of ICG fluorescence over the entire leg region, including PLNs and paws, was visualized under an infrared laser and recorded. The NIR imaging was repeated at 24 hours post ICG injection (suppl fig. 1). These images were read into the Image J software. Regions of interest (ROI) defining the PLNs and injection site were identified, yielding 4 outcome measures of lymphatic function: 1) T-initial (Ti), which is the time that it takes for the ICG to be detected in the draining PLN; 2) S-max, which is the maximum ICG signal intensity observed in the PLN during the first day imaging session; 3) T-max, which is the time it takes for a PLN to achieve maximal ICG signal intensity; and 4) % Clearance, which is an assessment of ICG wash out through the lymphatics and is quantified as the percent difference of ICG signal intensity between the two NIR scans from the ROI of the PLNs or footpad at 1) S-max (first day) and 2) 24 hours post ICG injection.
To observe the ICG distribution within the lymphatic network of PLNs, ICG was injected intra-dermally into footpads, and PLNs were harvested 1 hour later. Frozen sections (10 μm) were observed under a microscope with an infrared filter. After taking pictures to record the distribution of ICG fluorescence, the same section was stained with rabbit anti-Lymphatic Vessel Endothelial Receptor 1 (LYVE-1) antibody (Abcam Inc. Cambridge. MA) followed by Alexa Fluor 488 F(ab′)2 fragment goat anti-rabbit IgG (H+L), as we described previously (12).
Immunostaining and histomorphometric analysis
PLN, ankle, and knee specimens were fixed in formalin and embedded in paraffin. For immuno-fluorescence staining and data analysis, sections were stained with a mixture of PE-anti-CD11b or PE-anti-CD11c and anti-VEGF-C antibody followed by Alexa Fluor 488 goat anti-rabbit IgG and by To-Pro-3 iodide, as we described previously (12). Three pictures were taken from each section (x40) from different fields. The analysis was performed by counting the To-Pro-3 iodide+ cells as the total cell number in each field, and the numbers of CD11b+ and VEGF-C+ cells in the same field. The data were presented as the % of CD11b+ or VEGF-C+ cells over the total cell number. For immunohistochemistry staining, adjacent serial sections were stained with anti-LYVE-1 or anti-CD31 antibodies. Lymphatic vessels were quantified by a point-counting method, as we described previously (31). For each mouse, 2 sections were cut at 250 μm apart and the area and size of LYVE-1+ lymphatic vessels or CD31+ blood vessels were measured within the synovial tissue and expressed per square millimeter of synovium, as we previously described (12). The % of inflammatory area was measured from H&E-stained joint sections. The data were presented as the mean from 3 levels of each ankle joint.
Quantitative real time RT-PCR
RNA was extracted using TRIzol Reagent and cDNA synthesis was performed using GeneAmp RNA PCR core kit. Quantitative PCR amplification was performed with gene-specific primers (suppl table) using an iCycler real-time PCR machine and iQ SYBR Green supermix. The relative standard curve method was used to calculate the amplification difference for each primer set, as we described previously (32). The quantity of the target gene mRNA was then obtained by division of each value by the actin value. Standards and samples were run in triplicate.
Statistics
Data are presented as means ± standard deviation, and all experiments were performed at least twice with similar results. Statistical analyses were performed with Stat view statistical software. Differences between two groups were compared using unpaired Student t-test and more than two groups were compared using one-way ANOVA, followed by a Bonferroni/Dunnet test. p values less than 0.05 were considered to be statistically significant.
RESULTS
Increased lymphatic drainage in draining lymph nodes of TNF-Tg mice
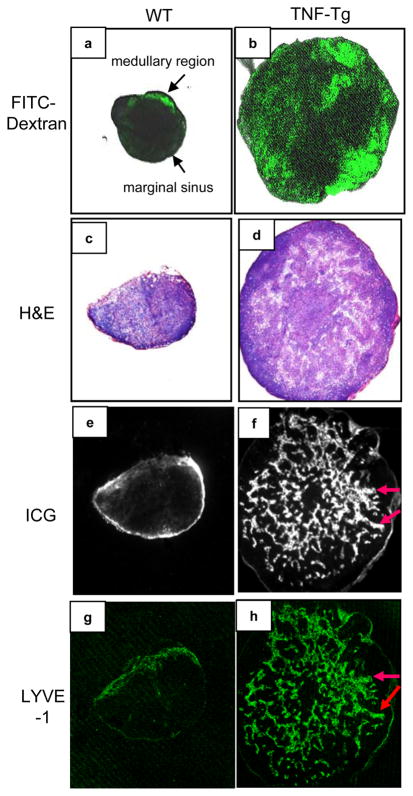
We reported previously that 4–6-month-old TNF-Tg mice have increased lymphangiogenesis in their PLNs, as evidenced by numerous dilated LYVE-1+ vessels (13). These large lymphatic vessels are associated with severe ankle arthritis and variable knee synovitis in all mice at this age. To determine if these dilated LYVE-1+ sinuses in the PLNs are functional and capable of draining afferent lymph, we intra-dermally injected FITC-dextran as a lymphatic tracer into the footpads of 4–6-month-old TNF-Tg mice and wild type (WT) littermate controls. The distribution of FITC fluorescence within the PLNs was observed by scanning confocal microscope. In WT mice, a thin layer of the FITC fluorescence was visualized primarily in marginal sinuses, which receives lymph from the afferent lymphatic vessels (Fig. 1a). The majority of the FITC fluorescence was concentrated at the medullary region, from which lymph exits through efferent lymphatic vessels (33). In contrast, the FITC signals were broadly distributed throughout the TNF-Tg PLNs (Fig. 1b). TNF-Tg PLNs were markedly expanded and displayed disorganized T and B cell zones compared to WT (Fig. 1c & d). The distribution pattern of actively draining lymph was corroborated by ICG-NIR imaging, as intra-dermal injection of ICG, another lymphatic tracer, into the footpad generated the same results as FITC-dextran (Fig. 1e & f). To confirm that the ICG was within lymphatic vessels we performed LYVE-1 immunostaining in adjacent sections (Fig. 1g & h), which demonstrated that ICG was contained within numerous dilated lymphatic sinuses. These findings indicate that chronic inflammation in the lower extremities of TNF-Tg mice leads to significantly increased lymphangiogenesis and functional dilation of lymphatic sinuses in the PLNs, which actively drain fluid from the foot.
Figure 1.
Increased lymphangiogenesis and lymphatic flow in draining popliteal lymph nodes of TNF-Tg mice. Popliteal lymph nodes (PLNs) from 4–6 month-old TNF-Tg mice and WT littermates (N=4) were used. All photos were taken at x4. FITC-dextran was injected intra-dermally into the footpads and PLNs were removed 1 hour later for confocal microscopy. Sixty to eighty slices were scanned from the surface of the nodes, and images 500μm deep from representative WT (a), and TNF-Tg (b) mice are shown. Indocyanine green (ICG) was injected intradermally into the footpads of WT (c, e, g) and TNF-Tg (d, f, h) mice, and PLNs were harvested 1 hour later for frozen section. Representative H&E sections show extensive enlargement of TNF-Tg PLN (d) vs WT PLN (c). Parallel sections were observed with an infrared filter, and representative images show the distribution of ICG fluorescence (e & f). The same sections were then stained with anti-LYVE-1 antibody and fluorescent images of LYVE-1+ staining are shown (g & h). The overlaping ICG and LYVE-1 signals are indicated by arrows.
Blockade of VEGFR-3 signal pathway prevents increased lymph node enlargement and lymphangiogenesis but increases the severity of joint synovitis in TNF-Tg mice
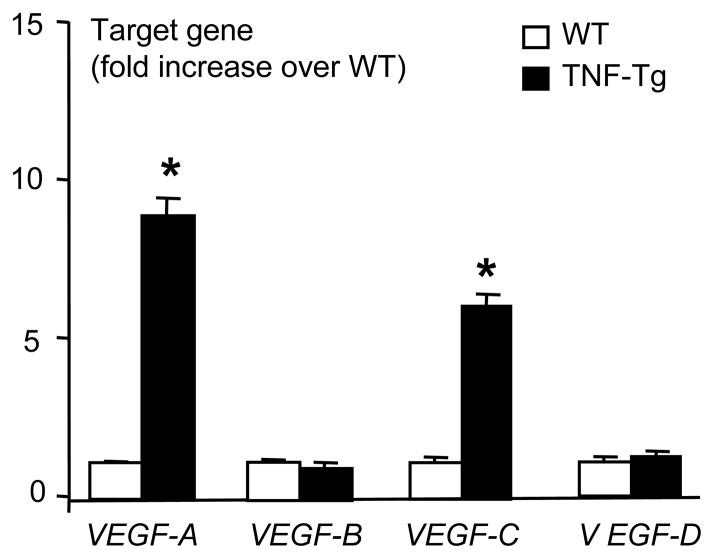
Inflammation-induced lymphangiogenesis in various animal models has been demonstrated to be mediated by inflammatory cytokine induced VEGF-A and/or VEGF-C signaling pathways (15–17, 34). To investigate the involvement of these VEGF members in our model, we performed quantitative RT-PCR on mRNA isolated from WT and TNF-Tg PLNs and examined the expression levels of VEGF-A, B, C, and D mRNA (Fig. 2). Consistent with other models, we found that VEGF-A and VEGF-C levels were significantly increased in TNF-Tg PLNs compared to WT nodes, while VEGF-B and VEGF-D levels were unchanged.
Figure 2.
Increased VEGF-A and VEGF-C expression in lymph nodes of TNF-Tg mice. Total RNA was extracted from pooled PLNs (n= 3–4) obtained from 4–6-month-old TNF-Tg mice or WT littermates, and the expression levels of VEGF family members were determined by real time RT-PCR standardized to a β-actin control. The fold changes were calculated by dividing the value of target gene over that of WT samples. Values are the mean ± SD of triplicate loadings (*p <0.05 vs. WT).
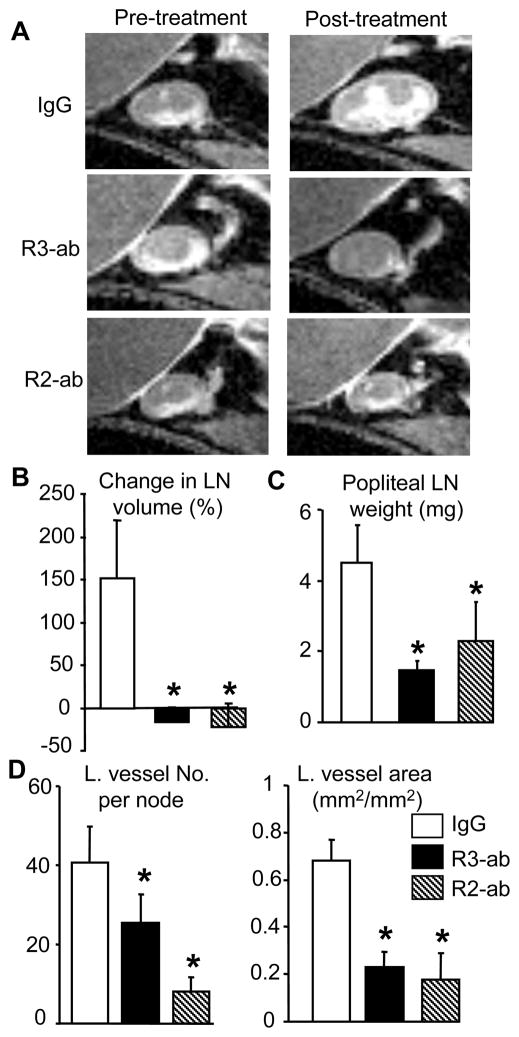
In order to further elucidate the roles of VEGF-A and VEGF-C signaling in TNF-induced inflammation and lymphangiogenesis during the development and progression of chronic arthritis in our model, we chose to perform VEGFR blockade experiments with neutralizing antibodies. Our hypothesis for these experiments was that blockade of VEGFR-2 would inhibit both inflammation and lymphangiogenesis by reducing angiogenesis as has been show for VEGF-A antagonism (15), while interruption of VEGF-C/VEGFR-3 signaling specifically blocks lymphangiogenesis, but not angiogenesis or inflammation (25). To test this hypothesis we treated 2.5 month-old TNF-Tg mice with anti-VEGFR-2, VEGFR-3 or irrelevant IgG control antibodies for 8 weeks, and assessed the treatment effects on PLN volume and lymphangiogenesis via CE-MRI obtained before and after therapy. Compared to the baseline values, the PLN volume in placebo-treated mice increased by more than 100%, while it was unchanged or slightly decreased in VEGFR-2 and VEGFR-3 antibody-treated mice (Fig. 3A & B). Accordingly, the PLNs in anti-VEGFR treated mice weighed significantly less than those of IgG-treated animals (Fig. 3C). LYVE-1 immunostaining and histomorphormetric analysis of the PLN sections revealed that VEGFR-2 and 3 antibodies significantly reduced the size, number, and area of LYVE-1+ lymphatic capillaries compared to placebo controls (Fig. 3D, suppl fig. 2).
Figure 3.
VEGFR-2 and VEGFR-3 neutralizing antibodies prevent TNF-induced PLN enlargement and lymphatic vessel formation. TNF-Tg mice (2.5-month-old, N=5/group) received CE-MRI of ankle and knee joints to obtain baseline value of synovial and PLN volume. Animals were then treated with VEGFR-2 or VEGFR-3 antibodies, or IgG (0.8 mg/mouse, i.p. 2/week) for 8 weeks. The post-treatment CE-MRI were performed one day before sacrifice. (A) Representative 2D CE-MRI images show a marked increase in IgG treated PLN volume over 8 weeks, compared to the lack of changes in PLN from antibody treated animals. (B) The % change in PLN volume from baseline is presented as mean ± SD for each treatment. (C) The PLN weights after 8-weeks are presented as mean ± SD for each treatment. (D) Histomorphometry of lymphatic vessel number and area in anti-LYVE-1 antibody-stained sections. The data are mean ± SD for each treatment.*p<0.05 vs. IgG.
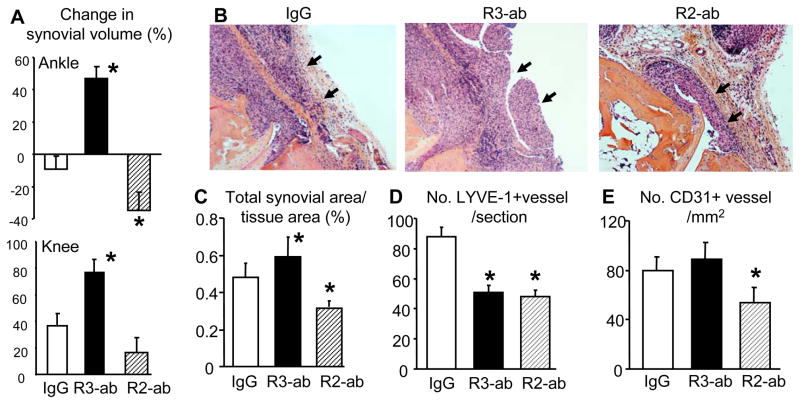
Having demonstrated the effects of VEGFR blockade on lymphangiogenesis in PLNs, we next examined its effects on joint inflammation to establish the direct relationship between lymphatic vessel formation and synovitis. 3D analyses of the CE-MRI data revealed dramatic differences in changes in ankle synovitis among all three treatment groups (Fig. 4A, suppl fig. 3). The synovial volumes of the placebo group demonstrated an insignificant change, those of the anti-VEGFR-3 group were significantly increased, and those of the anti-VEGFR-2 group were significantly decreased. The mean percent changes also reflected this, with a 45% increase in the anti-VEGFR-3 group and a 35% decrease in the anti-VEGFR-2 group. Volumetric assessment of knee synovitis also demonstrated that anti-VEGFR-3 treatment exacerbated inflammatory arthritis (Fig. 4A). These findings were corroborated by histomorphometric assessment of the inflammatory tissue in the ankle joints of H&E stained histology sections, which also demonstrated a significant increase and decrease in ankle inflammation in the VEGFR-3 vs. VEGFR-2 groups, respectively (Fig. 4B & C).
Figure 4.
Differential effects of VEGFR-2 and VEGFR-3 blockade on ankle synovial volume in TNF-Tg mice. (A) The ankle (upper panel) and knee (lower panel) synovial volumes of the mice described in Figure 3 before and after antibody treatment are shown as the mean ± SD percentage change from baseline (*p<0.05 vs. IgG). (B) Representative H&E stained sections (x10) of ankle tissues show that the anti-VEGFR-3 treatment increases while anti-VEGFR-2 treatment decreases inflammatory area, compared to IgG control (arrows). (C) Histomorphometry of inflammation area in H&E stained ankle sections is presented as the mean ± SD percentage of synovial area over the total tissue area (*p<0.05 vs. IgG). Immunostaining on parallel sections for LYVE-1+ lymphatic vessels (D) and CD31+ blood vessels (E) was performed, and the number of vessels is presented as mean ± SD for each treatment group (*p<0.05 vs. IgG).
As these results support our hypothesis regarding the differential effects of VEGFR-2 vs. VEGFR-3 blockade during chronic arthritis, we next investigated the changes in the vasculature within joints via immunohistochemistry. Consistent with our PLN data, both VEGFR-2 and VEGFR-3 antibody-treated mice had fewer LYVE-1+ lymphatic vessels compared to placebo treated controls (Fig. 4D). In contrast, only the anti-VEGFR-2 treatment reduced the number of CD31+ blood vessels (Fig. 4E), indicating that this treatment inhibits angiogenesis, an essential factor for inflammation. Collectively, our interpretation of these findings is that VEGFR-2-induced lymphangiogenesis is indirect through its stimulatory effects on angiogenesis and its recruitment of VEGF-C-producing inflammatory cells to the joint. This triggers VEGFR-3 signals to stimulate lymphangiogenesis in the synovium and draining lymph nodes.
Exacerbation of inflammatory arthritis by VEGFR-3 blockade is associated with decreased afferent lymph flow to draining nodes
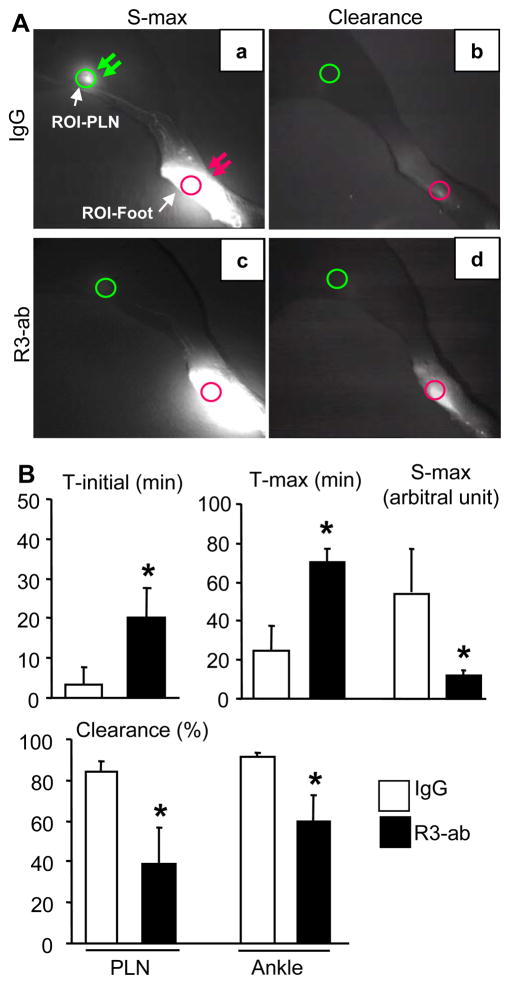
In order to test the hypothesis that VEGFR-3 signals are responsible for increased lymphatic drainage of inflamed joints during arthritis progression we performed ICG-NIR lymphatic imaging on the legs of TNF-Tg mice treated with anti-VEGFR-3 or placebo. Figure 5 demonstrates the dramatic differences of local lymphatic flow between placebo and anti-VEGFR-3 treatment after 8-weeks of therapy. At one hour after ICG injection, placebo PLNs displayed a bright fluorescent signal at S-max (Fig. 5A-a) compared to anti-VEGFR-3 treated PLNs (Fig. 5A-c). This indicates that less ICG was traveling to the PLNS through lymphatic vessels as a result of VEGFR-3 blockade reduced lymphangiogenesis. At 24 hours after ICG injection, placebo PLNs (Fig. 5A-b) and paws (Fig. 5A-d) had very little ICG fluorescence, indicating more ICG had been cleared away from these sites through the lymphatics. In contrast, at the same time point, the fluorescent signal in anti-VEGFR-3 treated paws remained strong, an indicator of slower ICG clearance. Quantitative analysis of the ICG-NIR images confirmed the significant increases in T-initial and T-max, and significant decreases in S-max and ICG clearance in anti-VEGFR-3 vs. placebo treated mice (Fig. 5B). Thus, these results demonstrate for the first time that VEGFR-3 induced lymphangiogenesis protects joints from accelerated arthritis by increasing the afferent flow of lymph from the inflamed tissues.
Figure 5.
VEGFR-3 blockade inhibits lymph flow from the foot to draining popliteal lymph nodes. TNF-Tg mice (2.5-month-old, N=4) were treated with VEGFR-3 neutralizing antibody or IgG as described in Figure 3, and received an ICG intra-dermal injection in their hind footpads after 8 weeks of treatment. Five min after the ICG administration the legs image for 1–2 hours until reaching the S-max using a NIR camera, and this NIR imaging was repeated for 10 min 24 hours later. (A) Representative ICG-NIR images obtained at S-max (a, c) and 24 hour after injection (b, d) show the gross difference in lymph drainage from the injection site in the footpad (red arrow) to the PLN (green arrows), in placebo (a, b) and anti-VEGFR-3 (c, d) treated mice. Circles are the regions of interest at the injection site where quantification was performed. (B) T-initial, S-max, T-max, and % Clearance in the region of interest (indicated by circles in A-a) were analyzed as described in Materials and Methods, and are presented as the mean ± SD (*p<0.05 vs. IgG placebo).
VEGFR-3 blockade decreases VEGF-C expressing CD11b+ cells in draining lymph nodes of TNF-Tg mice
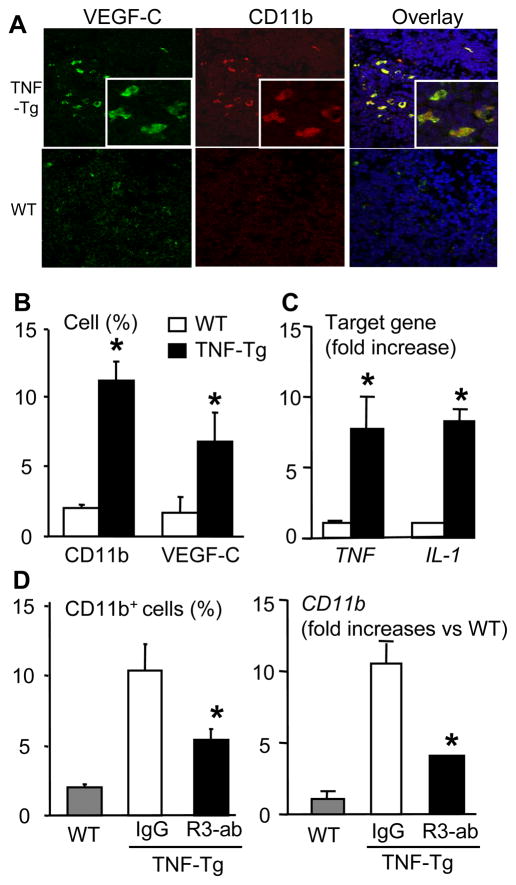
Since it is known that CD11b+ macrophages and dendritic cells are the primary infiltrating cells that produce VEGF-C in inflammatory tissues (17, 35), we investigated this cell population in PLNs of TNF-Tg mice with inflammatory arthritis vs. WT controls using two color immunofluorescence microscopy. The results demonstrated that the number of CD11b+ and VEGF-C+ cells was significantly increased in TNF-Tg PLNs vs. WT, and many of these cells were CD11b/VEGF-C double positive (Fig. 6A&B). The expression of TNF and IL-1 mRNA was increased in TNF-Tg PLNs (Fig. 6C). Interestingly, this CD11b+ population was markedly reduced in PLNs of anti-VEGFR-3 treated TNF-Tg mice as evidenced by a significant decrease in immunoreactive cells and CD11b transcript levels (Fig. 6D). These data indicate that increased expression of inflammatory cytokines and the presence of myeloid cells in draining lymph nodes may contribute to lymph node lymphangiogenesis in TNF-Tg mice through a VEGF-C/VEGFR-3 mediated event.
Figure 6.
VEGFR-3 blockade decreases VEGF-C-expressing CD11b+ cells in lymph nodes of TNF-Tg mice. (A) PLNs from TNF-Tg mice (4–6-month-old) and WT littermates were double immuno-stained with PE-anti-CD11b and FITC-anti-VEGF-C antibodies. Representative pictures taken at 40x indicate the co-localization of CD11b and VEGF-C proteins. (B) Histomorphometry was performed to determine the percentage of CD11b+ or VEGF-C+ cells in the PLNs. The values are the mean ± SD of 4 fields from 4 individual mice per genotype (*p<0.05 vs. WT). (C) Total RNA was extracted from pooled (n= 3–4) PLNs and the expression levels of TNF and IL-1 were determined by real-time RT-PCR standardized to a β-actin control. The data are presented as the mean fold-increase ± SD vs. the mean WT expression (*p<0.05 vs. WT). (D) PLNs from IgG- and VEGFR-3 neutralizing antibody-treated TNF-Tg mice were stained with anti-CD11b antibody, and the percentage of CD11b+ cells was calculated by dividing the number of CD11b+ cells by the total number of cells (left panel). The expression levels of CD11b mRNA were determined by real time RT-PCR (right panel). The data are presented as the mean fold-increase ± SD vs. the mean IgG expression. *p<0.05 vs. IgG treated mice.
DISCUSSION
Increased lymphatic vessel formation has been reported in several animal models of inflammation (34). However, it remains unclear if increased lymphangiogenesis only reflects the consequences of inflammation or if it plays an active role in the development and progression of inflammation. In the current study, we used TNF-Tg mice as a model of chronic inflammatory arthritis and demonstrated that blockade of VEGF-C/VEGFR-3 signaling by VEGFR-3 neutralizing antibody reduces lymphangiogenesis and lymphatic drainage and increases the severity of joint synovitis. In contrast, VEGFR-2 blockade reduces both lymphangiogenesis and synovitis. These findings indicate that lymphangiogenesis and lymphatic drainage actively contribute to the progression of inflammatory reactions in chronic inflammation. Improvement and maintenance of sufficient lymphatic drainage may represent a new therapeutic strategy for chronic inflammatory disorders.
Clinical studies in patients with RA have demonstrated high levels of pro-inflammatory cytokines in lymph draining arthritic joints and the enlargement of local draining lymph nodes in the ipsilateral limb (36, 37), indicating that local lymphatic circulation from inflamed joints to draining lymph nodes may affect the progression of inflammatory processes. It is predictable that lymph from arthritic joints and surrounding tissues carries large amounts of cytokines and immune cells. When this inflammatory lymph reaches lymph nodes, it could stimulate lymphangiogenesis. Thus inhibition of inflammation could reduce lymphangiogenesis. However, it is not clear whether or not lymphangiogenesis affects the natural progression of inflammation. This is an important point, because accumulated evidence from animal models (4, 12, 17) and clinical studies (8, 9, 14) has demonstrated increased lymphangiogenesis in inflammatory tissues.
Both VEGF-A and VEGF-C signaling pathways have been implicated in inflammatory lymphangiogenesis (38). VEGFR-2 transduces signals in blood and lymphatic endothelial cells, while VEGFR-3 mediates signaling only in lymphatic endothelial cells. Thus, VEGFR-3 blockade affects lymphatics specifically (25). We found that VEGFR-3 neutralizing antibody treatment of TNF-Tg mice reduces the number and area of joint and PLN lymphatic vessels, but it significantly increases the severity of joint inflammation. Decreased lymphangiogenesis is accompanied by reduced or slower transport of the lymphatic tracer, ICG, from paws to PLNs and reduced clearance of ICG from paws and PLNs. These findings strongly indicate that adequate lymph drainage from inflamed paws to local draining lymph nodes plays a beneficial role in the inflammatory process. Our findings are consistent with a recent report in which the same VEGFR-3 neutralizing antibody reduced the size of draining lymph nodes and increased lung weight, an outcome measure for lung inflammation, in a bacterial-induced airway inflammation model (17). Thus sufficient lymphatic trafficking between the primary inflammation sites and local draining lymph nodes may play an important role in limiting the progression of inflammation.
We found that in contrast to VEGFR-3 blockage, VEGFR-2 neutralizing antibody significantly reduces both joint inflammation and lymphangiogenesis, indicating that VEGFR-2 and VEGFR-3 signal pathways have different mechanisms for reducing inflammatory lymphangiogenesis. Our explanation is that VEGFR-2 blockade-induced reduction in lymphatics is likely indirect through its inhibitory effect on angiogenesis. VEGF-A or VEGFR-1 inhibition reduces joint damage in various models of arthritis (39, 40). However, a previous study reported no effects of VEGFR-2 neutralization on arthritis in K/BxN arthritic mice model (39). The discrepancy between our results and the previous report may reflect differences in the animal models used. K/BxN mice develop severe and aggressive joint lesions around 4 weeks of age (41, 42) and the disease is triggered following recognition of a NOD-derived MHC class II molecule by the transgenic TCR (43). The Tg3647 line used in our study develop arthritis at a much slower pace (22), and TNF is the pathogenic mediator. Thus, VEGFR-2 inhibition may be more effective in chronic inflammatory arthritis.
In this study, we started antibody treatment in 2.5-month-old TNF-Tg mice because our previous studies demonstrated that knee arthritis starts around this time and becomes more severe thereafter (22, 23), and we wanted to examine the effect of lymphatic blockade on the progression of joint lesions. Hayer et al have extensively characterized the initiation of ankle arthritis in this model, as well as its cellular make up (44). However, the timing of the peak synovitis is unknown. Using a newly developed murine dual RF receiver coil that allows us to image knee and ankle joints in a single MRI scan, our preliminary results indicate that ankle synovitis starts at 1.5 months of age and peaks at 2.5 months of age (synovial volume mm3: 12.5+3 in 1.5-month, 21+4.4 in 2.5 month and 21+3.4 in 4.5 month). In contrast, the synovial volume increases about 35% in the knee joints between 2.5 and 4.5 month of age (Fig. 4). These data suggest that the antibody treatment in this study likely started at the peak of the ankle synovitis and at the progression phase of the knee synovitis. Thus sufficient lymphatic function may not only slow the disease progression but also treat the disease. The effect of increased lymphangiogenesis on the joint lesions is currently under investigation”.
CD11b+ myeloid cells are the major source of VEGF-C in primary inflammatory sites and are responsible for promoting new lymphatic vessel formation. We found that in PLNs of TNF-Tg mice the majority of CD11b+ cells express VEGF-C, suggesting that VEGF-C in the lymph nodes is also produced by CD11b+ myeloid cells. Currently, the original of these CD11b+ cells is not clear. They may migrate from the blood circulation or through afferent lymphatics from joints or both. VEGFR3 antibody-treated mice have decreased CD11b+ cells in the PLNs. We believe that reduced CD11b+ cells in these mice is due to reduced CD11b+ cell drainage from the ankle and knee joints to the lymph nodes through lymphatic vessels because the antibody does not directly target these cells.
The mechanisms that regulate the recruitment and retention of CD11b+ cells in the PLNs are not known. The CCL19-CCL21/CCR7 axis is the major chemokine system expressing in the inflammatory lymphoid tissues and lymph nodes (45). CCL19/CCL21 is produced by non-hematopoietic stromal and fibroblast-like cells and attracts CCR7 expressing dendritic cells to the lymphoid tissues and lymph nodes (46, 47). CD11b+ cells are composed of dendritic cells and their precursors, thus whether or not the CCL19-CCL21/CCR7 axis is responsible for increased CD11b+ cells in the TNF-Tg PLNs needs to be studied in future. Furthermore, the chemokine, CXCR12, maintains the CD11b+ hematopoietic cells in peripheral organs after they have been recruited from bone marrow by VEGF-A (48) and bone marrow derived CD11b+ cells migrate to the CXCL12 gradients (49). In our preliminary study, we found that CXCR12 mRNA levels are increased in TNF-Tg mouse PLNs compared to WT PLNS (data not shown), suggesting that the CXCL12/CXCR4 axis may also be involved in retaining CD11b+ cells in TNF-Tg PLNs.
Based on our findings, we have proposed a model to explain the importance of the lymphatic system in the development and progression of inflammatory arthritis (supplemental figure 4). Joint inflammation recruits circulating CD11b+ myeloid cells from circulation. These cells produce lymphatic growth factors, such as VEGF-C, to stimulate lymphatic vessel formation. The functional lymphatic vessels transport inflammatory lymph carrying inflammatory cells, catabolic factors and cytokines to the draining lymph nodes and promote lymphangiogenesis, leading to an expansion of the lymph nodes and dilation of lymphatic sinuses containing inflammatory cells. Thus, sufficient lymphatic drainage could limit the degree of joint inflammation and the manipulation of the lymphatic system may represent a novel therapy for inflammatory disorders.
Supplementary Material
Acknowledgments
The authors would like to thank Edmund Kwok and Zhigang You for developing the dual coil; and Ms. Yan Lu, Xiaoyun Zhang and Yanyun Li for technical assistance with the histology. This work was supported by research grants from the National Institutes of Health PHS awards (AR48697 and AR53586 to LX, DE17096 and AR54041 to ES, DK075036 to RW). Part of Dr. Zhou, Quan’s salary was supported by a grant from the National Science Fund for Distinguished Young Scholars of China (30625043 to YJW).
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Lianping Xing has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Drs. Guo, Zhou, Proulx, Wood, Schwarz, and Xing.
Acquisition of data. Drs. Guo, Zhou, Proulx, Wood, and Xing.
Analysis and interpretation of data. Drs. Guo, Zhou, Proulx, Wood, Ji, Ritchlin, Wang, Pytowski, Zhu, Schwarz, Xing.
Manuscript preparation. Drs. Guo, Zhou, Proulx, Wood, Ji, Ritchlin, Pytowski, Zhu, Wang, Schwarz, and Xing.
Statistical analysis. Drs. Guo, Zhou, and Proulx.
References
- 1.Gerli R, Solito R, Weber E, Agliano M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology. 2000;33(4):148–57. [PubMed] [Google Scholar]
- 2.Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55(2):122–45. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- 3.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16(7):773–83. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 4.Xing L, Ji RC. Lymphangiogenesis, myeloid cells and inflammation. Expert Rev Clin Immunol. 2008;4(5):599–613. doi: 10.1586/1744666X.4.5.599. [DOI] [PubMed] [Google Scholar]
- 5.Makinen T, Alitalo K. Lymphangiogenesis in development and disease. Novartis Foundation Symposium. 2008;283:87–98. doi: 10.1002/9780470319413.ch8. [DOI] [PubMed] [Google Scholar]
- 6.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104(4):1048–57. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 7.Kerjaschki D. Lymphatic neoangiogenesis in renal transplants: a driving force of chronic rejection? J Nephrol. 2006;19(4):403–6. [PubMed] [Google Scholar]
- 8.Paavonen K, Mandelin J, Partanen T, Jussila L, Li TF, Ristimaki A, et al. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J Rheumatol. 2002;29(1):39–45. [PubMed] [Google Scholar]
- 9.Wauke K, Nagashima M, Ishiwata T, Asano G, Yoshino S. Expression and localization of vascular endothelial growth factor-C in rheumatoid arthritis synovial tissue. J Rheumatol. 2002;29(1):34–8. [PubMed] [Google Scholar]
- 10.Lee SH, Suh JS, Kim HS, Lee JD, Song J, Lee SK. MR evaluation of radiation synovectomy of the knee by means of intra-articular injection of holmium-166-chitosan complex in patients with rheumatoid arthritis: results at 4-month follow-up. Korean J Radiol. 2003;4(3):170–8. doi: 10.3348/kjr.2003.4.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44(3):541–9. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Lu Y, Proulx ST, Guo R, Yao Z, Schwarz EM, et al. Increased lymphangiogenesis in joints of mice with inflammatory arthritis. Arthritis Res Ther. 2007;9(6):R118. doi: 10.1186/ar2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proulx ST, Kwok E, You Z, Beck CA, Shealy DJ, Ritchlin CT, et al. MRI and quantification of draining lymph node function in inflammatory arthritis. Ann N Y Acad Sci. 2007;1117:106–23. doi: 10.1196/annals.1402.016. [DOI] [PubMed] [Google Scholar]
- 14.Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP, et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis. 2008;67(11):1610–6. doi: 10.1136/ard.2007.083394. [DOI] [PubMed] [Google Scholar]
- 15.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110(9):3158–67. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115(2):247–57. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, et al. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol. 2008;294(3):H1326–34. doi: 10.1152/ajpheart.00900.2007. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72(3):161–71. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115(9):2316–9. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170(4):1178–91. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Schwarz EM, O’Keefe RJ, Ma L, Looney RJ, Ritchlin CT, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50(1):265–76. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 23.Proulx ST, Kwok E, You Z, Papuga MO, Beck CA, Shealy DJ, et al. Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance imaging and microfocal computed tomography. Arthritis Rheum. 2007;56(12):4024–37. doi: 10.1002/art.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witte L, Hicklin DJ, Zhu Z, Pytowski B, Kotanides H, Rockwell P, et al. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17(2):155–61. doi: 10.1023/a:1006094117427. [DOI] [PubMed] [Google Scholar]
- 25.Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97(1):14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 26.Childs LM, Paschalis EP, Xing L, Dougall WC, Anderson D, Boskey AL, et al. In vivo RANK signaling blockade using the receptor activator of NF-kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J Bone Miner Res. 2002;17(2):192–9. doi: 10.1359/jbmr.2002.17.2.192. [DOI] [PubMed] [Google Scholar]
- 27.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60(16):4324–7. [PubMed] [Google Scholar]
- 28.Leu AJ, Berk DA, Yuan F, Jain RK. Flow velocity in the superficial lymphatic network of the mouse tail. Am J Physiol. 1994;267(4 Pt 2):H1507–13. doi: 10.1152/ajpheart.1994.267.4.H1507. [DOI] [PubMed] [Google Scholar]
- 29.Sevick-Muraca EM, Sharma R, Rasmussen JC, Marshall MV, Wendt JA, Pham HQ, et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246(3):734–41. doi: 10.1148/radiol.2463070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon S, Sevick-Muraca EM. Noninvasive quantitative imaging of lymph function in mice. Lymphat Res Biol. 2007;5(4):219–31. doi: 10.1089/lrb.2007.1013. [DOI] [PubMed] [Google Scholar]
- 31.Boyce BF. Bone biopsy and histomorphometry in metabolic bone disease. In: Stevenson JC, editor. New techniques in metabolic bone disease. London: Butterworths; 1990. pp. 110–131. [Google Scholar]
- 32.Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem. 2000;278(2):175–84. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- 33.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology. 6. Garland Science Publishing; New York: 2005. The Immune System in Health & Disease. Chapter 1:Basic Concepts in Immunology. [Google Scholar]
- 34.Ji RC. Lymphatic endothelial cells, inflammatory lymphangiogenesis, and prospective players. Curr Med Chem. 2007;14(22):2359–68. doi: 10.2174/092986707781745541. [DOI] [PubMed] [Google Scholar]
- 35.Xing L, Schwarz E. Circulating osteoclast precursors: a mechanism and a marker of erosive arthritis. Curr Rheumatol Rev. 2005;1:21–28. [Google Scholar]
- 36.Motulsky AG, Weinberg S, Saphir O, Rosenberg E. Lymph nodes in rheumatoid arthritis. AMA Arch Intern Med. 1952;90(5):660–76. doi: 10.1001/archinte.1952.00240110086009. [DOI] [PubMed] [Google Scholar]
- 37.Cambiaggi G. Lymph nodes in rheumatoid arthritis; lymph node hyperplasia of Symmers’ type in a case of Still’s disease. Progr Med (Napoli) 1954;10(16):488–91. [PubMed] [Google Scholar]
- 38.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–60. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 39.De Bandt M, Ben Mahdi MH, Ollivier V, Grossin M, Dupuis M, Gaudry M, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J Immunol. 2003;171(9):4853–9. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- 40.Mould AW, Scotney P, Greco SA, Hayward NK, Nash A, Kay GF. Prophylactic but not therapeutic activity of a monoclonal antibody that neutralizes the binding of VEGF-B to VEGFR-1 in a murine collagen-induced arthritis model. Rheumatology (Oxford) 2008;47(3):263–6. doi: 10.1093/rheumatology/kem369. [DOI] [PubMed] [Google Scholar]
- 41.Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25(1):79–90. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]
- 42.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10(4):451–61. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286(5445):1732–5. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 44.Hayer S, Redlich K, Korb A, Hermann S, Smolen J, Schett G. Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis. Arthritis Rheum. 2007;56(1):79–88. doi: 10.1002/art.22313. [DOI] [PubMed] [Google Scholar]
- 45.Page G, Miossec P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J Pathol. 2004;204(1):28–38. doi: 10.1002/path.1607. [DOI] [PubMed] [Google Scholar]
- 46.Manzo A, Bugatti S, Caporali R, Prevo R, Jackson DG, Uguccioni M, et al. CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am J Pathol. 2007;171(5):1549–62. doi: 10.2353/ajpath.2007.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL, et al. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007;56(8):2492–502. doi: 10.1002/art.22748. [DOI] [PubMed] [Google Scholar]
- 48.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, Guo R, Schwarz EM, Boyce BF, Xing L. TNF inhibits production of stromal cell-derived factor 1 by bone stromal cells and increases osteoclast precursor mobilization from bone marrow to peripheral blood. Arthritis Res Ther. 2008;10(2):R37. doi: 10.1186/ar2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.