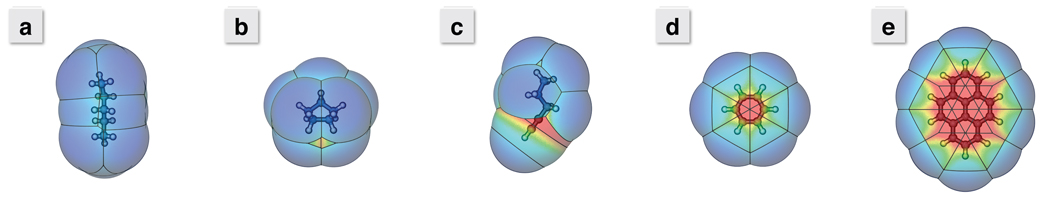
Figure 4.
Maps of the collective dispersion attraction about the solvent accessible surface (SAS) of a) n-pentane, b) cyclopentane, c) pent-1-yne, d) benzene, and e) pyrene. The color of the surface indicates the LJ well-depth, with blue starting at 0 kcal/mol and red lowering to deeper than 5 kcal/mol. Note the red “hot spots” around the triple bond in pent-1-yne and in the center of the benzene and pyrene ring planes. These indicate a significant enhancement of dispersion attraction with the surroundings. As these regions grow with increasing molecule size, these collective dispersion attractions will offset the cost of cavity formation in surrounding solvent. With a simple γA, all these surfaces would be a uniform blue.