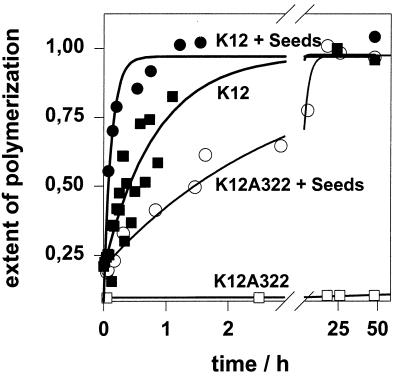
Figure 4.
Nucleation and growth are both dependent on dimeric tau protein. Monomeric tau (20 μM; K12A322) or 10 μM dimeric tau (K12 crosslinked via disulfide linkage of Cys322) was incubated in assembly buffer with 5 μM heparin in the absence or presence of seeds (corresponding to 1 μM tau protein). The time course of assembly shows that seeding accelerates assembly in both cases. Note that dimeric K12 without seeds is even faster than monomeric K12A322 in the presence of seeds, illustrating a high tendency of dimeric tau to self-assemble. The final extent of assembly is the same in all cases.