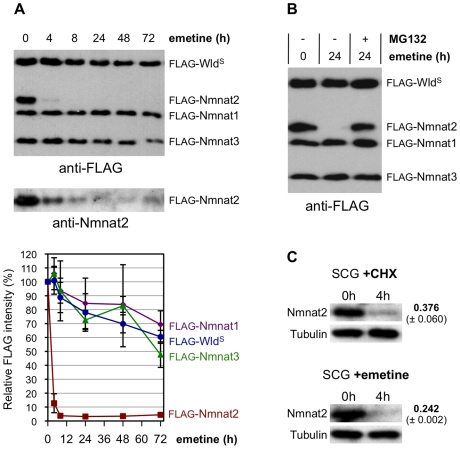
Figure 6. Nmnat2 is the most labile Nmnat isoform and is degraded by the proteasome.
(A) Relative stabilities of FLAG-tagged Nmnat isoforms and WldS in HEK 293T cells co-transfected with expression vectors for each and treated 24 h after transfection with 10 µM emetine to block translation for the times indicated. A representative FLAG immunoblot is shown (top panel). The same blot was re-probed with an Nmnat2 antibody (bottom panel) to show that loss of anti-FLAG signal is primarily due to protein turnover rather than cleavage of the FLAG epitope from the protein. Quantification of band intensities for each protein from three independent experiments is shown below as a percentage of untreated (0 h) band intensities (error bars = ±S.E.M.). Co-transfection allows direct comparison of stabilities of each protein in the same cells. (B) FLAG-Nmnat2 turnover in transfected HEK 293T cells is prevented by proteasome inhibition. Cells were transfected as in (A) and treated with 10 µM emetine for 0 or 24 h, ±20 µM MG-132. A FLAG immunoblot representative of three independent experiments is shown. (C) Endogenous Nmnat2 is rapidly turned over in SCG explant cultures after blocking translation with CHX (1 µg/ml) or emetine (10 µM). Representative immunoblots are shown comparing steady-state levels of Nmnat2 (0 h) with levels after 4 h of protein synthesis suppression. ß-Tubulin acts as a loading control. Nmnat2 band intensity at 4 h is shown as a fraction of that at 0 h after normalization to ß-Tubulin and was quantified from two independent experiments each (error bars = ±S.E.M.). These values are consistent with the different rates at which CHX (1 µg/ml) and emetine induce neurite degeneration (Figure 1).