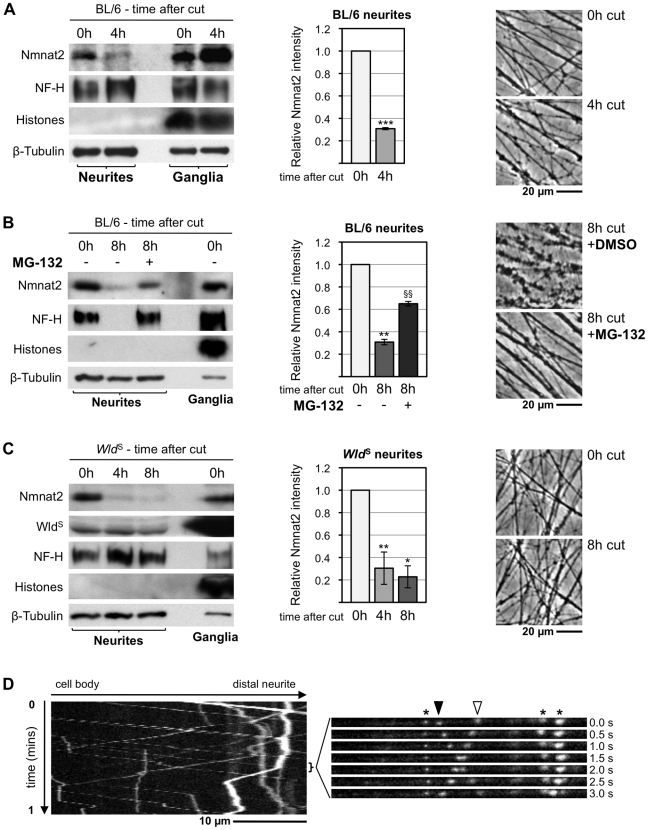
Figure 7. Endogenous Nmnat2 is present in SCG neurites and undergoes rapid turnover after transection.
(A–C) Relationship between Nmnat2 turnover in neurites and other parameters of neurite health in wild-type cultures (A), wild-type cultures ±20 µM MG-132 (B), and Wld S cultures (C). Representative immunoblots show detection of Nmnat2, WldS (where applicable), NF-H, 16 kDa core Histones, and ß-Tubulin just after cut (0 h) and 4 and/or 8 h later. Material collected from SCG explant cultures derived from 15–20 ganglia was needed to detect Nmnat2 in each lane. Loss of the NF-H band is an early consequence of axon degeneration and absence of the 16 kDa core Histones band in neurite extracts confirms there is no detectable contamination with SCG cell bodies or non-neuronal cells. ß-Tubulin represents a loading control. Relative Nmnat2 band intensities in neurite-only lanes are shown as a fraction of levels at 0 h after normalisation to ß-Tubulin and were quantified from two or three independent experiments (error bars = ±S.E.M.). Nmnat2 is significantly depleted in untreated wild-type and Wld S neurites shortly after separation from their ganglia (*p<0.05, **p<0.01, ***p<0.001, t test 4 or 8 h versus 0 h). Proteasome inhibition with MG-132 significantly reduces this Nmnat2 loss at 8 h after transection (§§ p<0.01, t test 8 h +MG-132 versus 8 h untreated). Images show representative transected neurite morphology (in the same field where applicable) at the indicated times after cut. (D) Kymograph of the bottom axon in Video S1 showing fast axonal transport of particles containing Nmnat2-eGFP with a bias in the anterograde direction. An image series from the indicated region of the kymograph is shown on the right. It highlights a particle moving anterogradely (filled arrowhead), one moving retrogradely (empty arrowhead), and several stationary particles (asterisks).