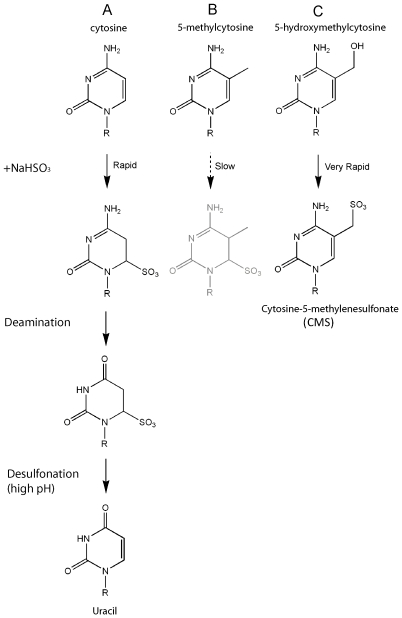
Figure 1. Reaction of sodium bisulfite with C, 5-mC and 5-hmC.
(A) Bisulfite-mediated deamination of cytosine. HSO3 − reversibly and quickly adds across the 5,6 double bond of cytosine, promoting deamination at position 4 and conversion to 6-sulfonyluracil. 6-sulfonyluracil is stable under neutral conditions, but is easily desulfonated to uracil (U) at higher pH. (B) 5-methylcytosine is deaminated to thymine by bisulfite conversion, but the rate is approximately two orders of magnitude slower than that of cytosine. (C) Bisulfite quickly converts 5-hydroxymethylcytosine to form cytosine-5-methylenesulfonate (CMS). This adduct does not readily undergo deamination [45].