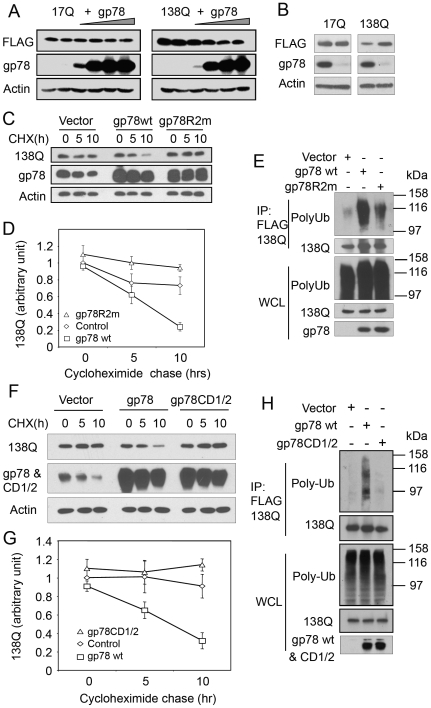
Figure 8. gp78 ubiquitinates and targets Nhtt138Q but not Nhtt17Q for degradation dependent on its RING finger and cue domain.
A. Overexpression of gp78 decreases the levels of Nhtt138Q but not Nhtt17Q protein. Increasing amounts of gp78 were expressed in HEK293 cells that had been transfected with Nhtt17Q or Nhtt138Q. 24 h after gp78 transfection, cells were processed for immunoblotting for the indicated proteins. B. Knockdown of gp78 stabilizes Nhtt138Q but not Nhtt17Q. gp78 was knocked down by RNAi in HEK293 cells expressing Nhtt138Q or Nhtt17Q. 2 days after the knockdown cells were processed for immunoblotting for gp78 and Nhtt17Q and Nhtt138Q. Actin was blotted as a loading control. C. gp78 enhances Nhtt138Q degradation, which requires an intact RING finger. HEK293 cells transfected as indicated were subjected to cycloheximide (CHX) chase. Nhtt138Q degradation was quantified and expressed as mean +/− SD, n = 3, in D. E. gp78 ubiquitinates Nhtt138Q. Nhtt138Q-expressing HEK293 cells were transfected with wt gp78, or gp78R2m, or empty vector as a control. Nhtt138Q was immunoprecipitated with anti-FLAG antibody. Precipitates were processed for immunoblotting for ubiquitin. F - H. Cue domain is required for gp78 to ubiquitinate and target Nhtt138Q for degradation. Wt gp78, gp78CD1/2, or empty vector-transfected HEK293 cells were subjected to analysis as described in C–E.