Abstract
Previous studies have implicated DTNBP1 as a schizophrenia susceptibility gene and its encoded protein, dysbindin, as a potential regulator of synaptic vesicle physiology. In this work, we found that endogenous levels of the dysbindin protein in mouse brain are developmentally regulated, with higher levels observed during embryonic and early postnatal ages than in young adulthood. We obtained biochemical evidence indicating that the bulk of dysbindin from brain exists as a stable component of biogenesis of lysosome-related organelles complex-1 (BLOC-1), a multi-subunit protein complex involved in intracellular membrane trafficking and organelle biogenesis. Selective biochemical interaction between brain BLOC-1 and a few members of the SNARE superfamily of proteins that control membrane fusion, including SNAP-25 and syntaxin 13, was demonstrated. Futhermore, primary hippocampal neurons deficient in BLOC-1 displayed neurite outgrowth defects. Taken together, these observations suggest a novel role for the dysbindin-containing complex, BLOC-1, in neurodevelopment, and provide a framework for considering potential effects of allelic variants in DTNBP1 – or in other genes encoding BLOC-1 subunits – in the context of the developmental model of schizophrenia pathogenesis.
Keywords: schizophrenia, DTNBP1, pallidin, syntaxin, synaptosomal-associated protein, biological plausibility, primary cell culture, neurite extension, susceptibility gene
Introduction
Schizophrenia is a psychiatric disorder with a prevalence of 0.5–1.0%.1 Although its heritability has been estimated to be in the order of 80%, unambiguous identification of susceptibility genes has proven to be extremely challenging.2 Nevertheless, several candidate genes have emerged and become the subject of intense multidisciplinary research, including follow-up genetic studies (e.g., replication attempts in independent patient cohorts) and basic experimentation aimed at establishing the “biological plausibility” of the proposed genetic association, i.e., whether the function of the gene product would be compatible with a role in schizophrenia pathogenesis.1
This paper is devoted to dysbindin, the product of the DTNBP1 gene.3,4 Genetic association between haplotype variants within DTNBP1 and increased risk of developing schizophrenia stemmed from a family-based study5 that was followed by more than two dozen studies.6,7 Such genetic association, however, is not unequivocal,8,9 and the possibility remains that the impact of DTNBP1 as a schizophrenia susceptibility gene might be restricted to the families involved in the initial studies5,10 and/or a rare form of childhood-onset psychosis.11 Although none of the DTNBP1 variants proposed to increase disease risk would modify the amino acid sequence of the encoded dysbindin protein, studies in postmortem brain samples have suggested a mechanism whereby these variants would act as cis-elements to decrease dysbindin mRNA levels.12–14 Furthermore, a significant reduction in the levels of the dysbindin protein was observed in postmortem brain regions from two independent cohorts of schizophrenic patients.15 Strikingly, such reduction in protein levels was more common among patients (73–93%) than expected from the frequency of the proposed susceptibility alleles,15 thus raising the possibility that the involvement of the dysbindin protein in the pathogenesis of schizophrenia could be more widespread than anticipated from studies that focus on DTNBP1 gene variants.
Recent studies on the sandy mouse line have provided additional support to the notion that altered dysbindin function may contribute to the risk of developing schizophrenia. The sandy mice, which arose from spontaneous mutation in a DBA/2J stock and carry a Dtnbp1 allele encoding a protein with an in-frame 22-residue deletion,16 displayed abnormal behavioral phenotypes consistent with deficits in social interaction and long-term and working memory.17–21 Reduced dopamine levels,17 and abnormalities in synaptic morphology and function,22 were also documented. These important findings notwithstanding, the function of dysbindin in the brain remains unclear. Although its initial description3 as a dystrobrevin-binding protein and a component of the dystrophin-dystroglycan complex had suggested a postsynaptic site of action, subsequent studies cast doubt on the in vivo significance of the dysbindin-dystrobrevin interaction23 and instead focused on possible roles for dysbindin at the presynaptic level.15,18,22,24–27 For instance, dysbindin was proposed to regulate synaptic vesicle biogenesis and release,22,24,25 an idea consistent with the hypothesis that schizophrenia is primarily a synaptic transmission disorder.28 However, another prevailing view is the so-called developmental model of schizophrenia pathogenesis, whereby genetic variants contribute with environmental factors to a developmental disruption of neural connectivity, embryonically or early in postnatal life (e.g., subtle defects in neuronal migration, dendritic arborization or axonal growth) or during adolescence (e.g., excessive pruning).1,29,30 To our knowledge, a putative role for dysbindin in brain development has not yet been investigated.
In this paper, we report that the dysbindin protein is expressed embryonically in mouse brain and is significantly more abundant during embryonic and early postnatal development than in adulthood. We also provide evidence that brain dysbindin exists as a stable component of BLOC-1 (biogenesis of lysosome-related organelles complex-1), a multi-subunit protein that in non-neuronal cells has been implicated in organelle biogenesis and intracellular membrane trafficking.31–37 Consistent with a potential role in neurodevelopment, results reported herein indicate that BLOC-1 from brain is capable of interacting with a subset of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)38 proteins previously implicated in neurite outgrowth, and that primary hippocampal neurons deficient in BLOC-1 display neurite extension defects.
Materials and methods
Information about recombinant proteins, antibodies, animals, primary cell culture, preparation of brain tissue extracts and SNARE complexes, and immunoblotting analysis is provided as Supplementary materials and methods.
In vitro binding assay
In a typical experiment, frozen bovine brain cytosol (~5 g/l total protein) was quickly thawed and diluted with an equal volume of 10 mM Hepes (pH 7.4), 0.15 M KCl, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM MgCl2 and protease inhibitor mixture [1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 10 mg/l leupeptin, 5 mg/l aprotinin and 1 mg/l pepstatin A]. In experiments aimed at testing the ability of cypin to bind BLOC-1, bovine brain cytosol was also used with minimal dilution upon addition of EDTA (to 1 mM) and of fresh dithiothreitol (1 mM) and protease inhibitor mixture. The diluted cytosol was then cleared by ultracentrifugation at 120000 × g for 30 min and/or filtration through a 0.45 μm filter (to remove any protein aggregates), and then by incubation with empty Glutathione-Sepharose 4 Fast Flow beads (GE Healthcare, Piscataway, NJ, USA). Purified glutathione-S-transferase (GST)-fusion proteins were immobilized on glutathione-Sepharose beads, while purified His6-S-tag-fusion proteins and recombinant SNARE complexes were immobilized on Protein-S-agarose beads (EMD Biosciences Novagen, Madison, WI, USA); in experiments using proteins immobilized on both types of beads, all samples contained a 1:1 (v/v) bead mixture to achieve comparable levels of non-specific protein binding. The immobilized recombinant proteins were then incubated with cleared cytosol at 4°C for at least 1 h, after which the beads were washed three times with ice-cold washing buffer (10 mM Hepes, pH 7.4, 75 mM KCl, 1 mM EGTA, 0.5 mM MgCl2) containing 0.1% (w/v) Triton X-100 and once with the same buffer lacking Triton X-100. Proteins bound to the washed beads were eluted by boiling in the presence of SDS-PAGE sample buffer, and subsequently analyzed by immunoblotting.
Immunocytochemistry and neurite outgrowth assay
Primary hippocampal neurons (see Supplementary materials and methods) were fixed, permeabilized and immunostained with antibodies to GAP43, βIII-tubulin and GFAP, as previously described.39 Digital images of randomly selected microscopic fields were acquired on a Zeiss Axioskop 2 microscope (Carl Zeiss) equipped with an ORCA-ER digital camera (Hamamatsu), using a 40× objective and appropriate filters. The images were saved using coded file names to mask the identity of cell samples, such that the person(s) involved in subsequent image analysis could not establish the identity of the samples. Image-based analysis of neurite number and length was performed using the Carl Zeiss AxioVision software. Processes that were shorter than 5 μm were excluded from the analysis.
Statistical analysis
Differences between steady-state protein expression levels were analyzed by regression analysis or one-way ANOVA followed by Bonferroni’s multiple comparison tests. For the analysis of data resulting from neurite outgrowth experiments, we focused on three measures per cell: neurite number, length of the longest neurite, and sum of lengths of all neurites. The significance of effects of BLOC-1 deficiency (in the pallid mutant mice) on these measures was tested using an ANOVA model in which “genotype” (wild type or pallid) was used as explanatory factor together with “days in culture” (1 or 3 days) and “primary culture preparation” (four independent preparations using serum-free Tii medium and one using DMEM plus serum; see Supplementary Table S1 for details). Mean values per digital image, rather than individual values per cell, were analyzed to minimize correlation between observations, and data were subjected to logarithmic transformation prior to ANOVA in order to make the assumption of Gaussian distributions appropriate. To investigate day-specific effects, data obtained for cells cultured for 1 or 3 days were analyzed separately, keeping genotype and primary culture preparation as the explanatory factors. Only P values associated with genotype as a factor are reported. Since several ANOVA tests were performed, to evaluate three types of measures and to investigate day-specific effects, the significance threshold was lowered to 0.01.
Results
Dysbindin protein levels in the mouse brain are developmentally regulated
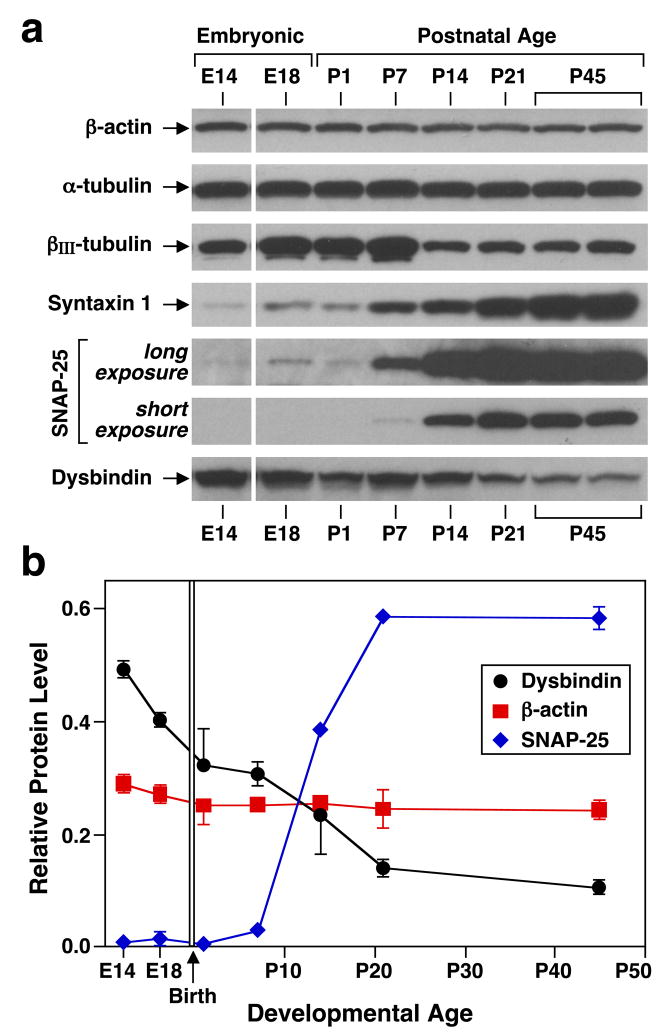
To investigate dysbindin protein levels in mouse brain and their possible developmental regulation, “whole-tissue” extracts prepared from mouse cerebral cortex at different ages, spanning from embryonic day (E)14 through postnatal day (P)45, were analyzed by immunoblotting. We used an affinity-purified, rabbit polyclonal antibody against dysbindin previously generated and validated.33 The antibody recognized a major protein species with an apparent molecular mass of ~50 kDa, a value which is in agreement with previous reports.3,15,26 As shown by representative immunoblots (Figure 1a) and quantitative analyses (Figure 1b), there was a consistent decline in dysbindin protein levels throughout the age period examined, unlike the roughly constant levels of housekeeping proteins (e.g., β-actin) or the dramatic increase in expression exhibited by synaptosomal-associated protein (SNAP)-25 and syntaxin 1. Such age-dependent decline in dysbindin protein levels was not unique to cerebral cortex; as shown in Figure 2, the average level in hippocampus from P1 mice was, like in cerebral cortex, more than three times that from P45 mice, and in cerebellum from P7 mice it was about twice that in cerebellum from P45 animals. These changes remained statistically significant upon normalizing the dysbindin protein level of each sample to that of β-actin (data not shown). Since we were concerned that these apparent declines in dysbindin abundance could be secondary to differences in cell composition of the dissected tissues, specifically due to gliogenesis, purified primary cultures of astrocytes, oligodendrocytes and neurons were examined for dysbindin expression. Both types of glial cells displayed steady-state levels of dysbindin that were comparable to, if not higher than, those detected in neurons (Supplementary Figure S1). Importantly, dysbindin protein levels also declined in primary cortical neurons kept in culture for longer than a week (Supplementary Figure S1). These results suggested that dysbindin protein expression in brain is developmentally regulated, implying a potential role for the protein in embryonic and early postnatal development.
Figure 1.
Developmental regulation of dysbindin protein level in mouse cerebral cortex. (a) Immunoblotting analysis of “whole-tissue” detergent extracts (20 μg total protein) prepared from cerebral cortices dissected out of C57BL/6J mice at embryonic day (E)14 and E18 and postnatal day (P)1 through P45. (b) Quantitative analyses of the relative expression levels of selected proteins in cortex from mice at different developmental ages. Each value derived from densitometric analysis was corrected for background and divided by the sum of values obtained in the same immunoblot image for one sample each of P1, P7, P14 and P21. For the sake of clarity, data are represented as means ± s.e.m. (2–4 independent samples per age), even when all data points were analyzed together by linear regression to assess departure from a theoretical line with slope equal to zero (18 degrees of freedom; dysbindin, P < 0.001; β-actin, not significant).
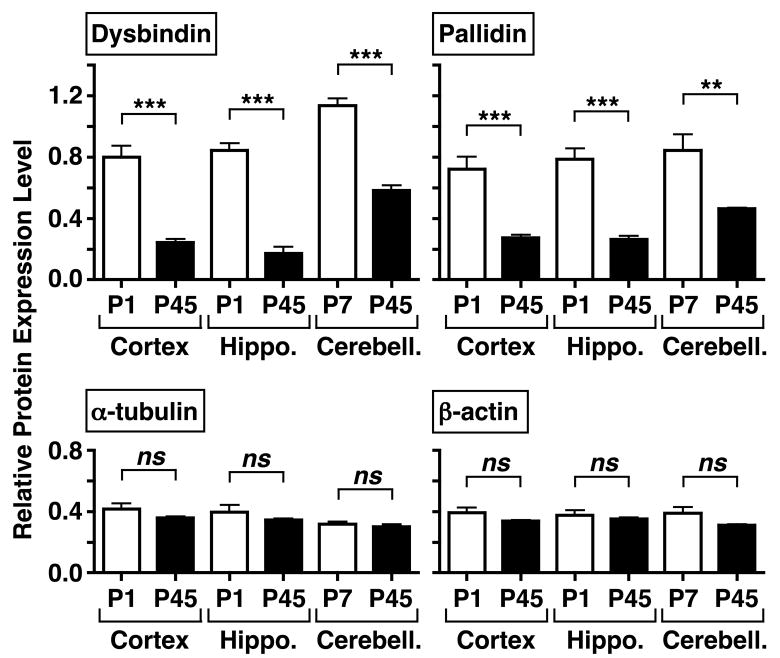
Figure 2.
Dysbindin and pallidin protein levels are higher in the cerebral cortex and hippocampus (Hippo.) of C57BL/6J mice at postnatal day (P)1, and in cerebellum (Cerebell.) from P7 mice, than in the corresponding brain regions of young adult (P45) mice. “Whole-tissue” detergent extracts were analyzed by immunoblotting using antibodies against the indicated proteins. The immunoreactivity signals derived from densitometric analysis were corrected for background and expressed as a ratio to the sum of background-corrected signals obtained in the same immunoblot for one sample each of the three brain regions of P45 mice. Each bar represents mean value ± s.e.m. of 4–5 independent brain samples. One-way ANOVA followed by Bonferroni’s multiple comparison tests: ** P < 0.01, *** P < 0.001, ns, not significant.
Brain dysbindin is a stable component of BLOC-1
To begin to understand which role(s) dysbindin may play during neurodevelopment, it was important to address the biochemical properties of the endogenously expressed protein. In non-neuronal cells, dysbindin associates into a multi-subunit complex, BLOC-1, which also contains the proteins pallidin, muted, cappuccino, snapin and BLOC-1 subunit (BLOS)1, 2 and 3.16,31–33,40,41 We had previously reported co-immunoprecipitation of endogenous dysbindin and pallidin from brain,23 and another group subsequently showed co-immunoprecipitation of dysbindin and snapin.26 To test whether these results reflected transient interactions of monomeric dysbindin with the other two proteins or stable association of dysbindin into a brain form of BLOC-1, two sets of experiments were performed:
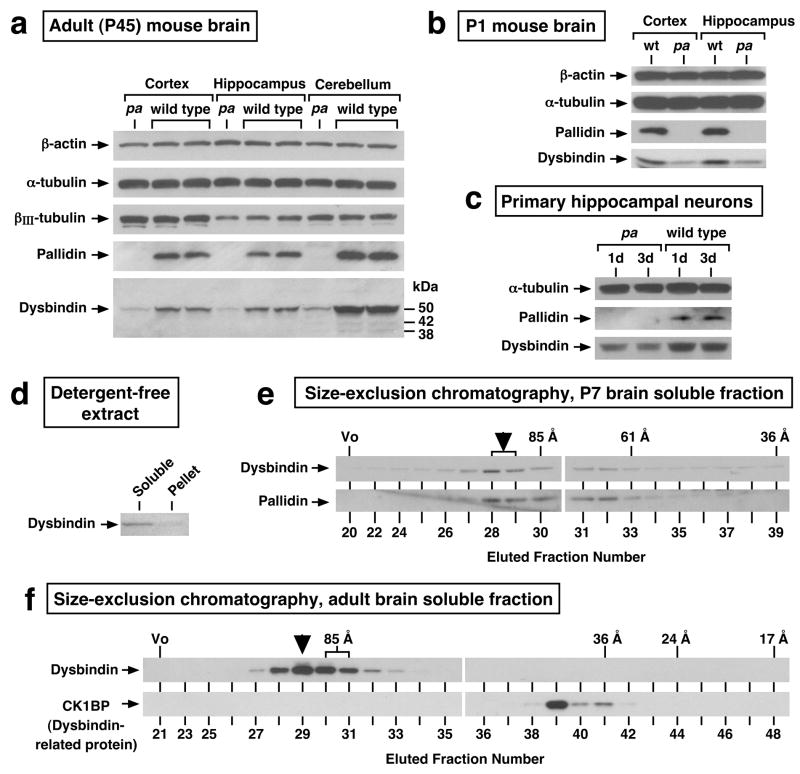
In the first set, the steady-state levels of the dysbindin protein were examined in selected brain regions from pallid mutant mice. These mice carry a non-sense mutation in the gene encoding pallidin,42 which is essential for BLOC-1 assembly.31,32 We reasoned that dysbindin protein levels would be significantly reduced in pallid mouse brain if the protein normally existed as a component of BLOC-1 and was biochemically unstable in unassembled form. Such is a usual phenomenon for subunits of stable protein complexes, and it has been observed for dysbindin from kidney16 and liver.33 As shown in Figure 3, panels a and b, drastically reduced levels of the major ~50-kDa species (and, where detectable, of two minor species of 42 and 38-kDa) were observed in “whole-tissue” extracts prepared from cerebral cortex, hippocampus and cerebellum of P45 pallid mice (Figure 3a), as well as from P1 pallid cerebral cortex and hippocampus (Figure 3b), as compared to equal total protein loads of the corresponding extracts from wild-type mice. Similar results were obtained at other ages (e.g., P7 cerebral cortex, hippocampus and cerebellum; data not shown) as well as in primary cultures of hippocampal neurons from P0 mice (Figure 3c).
Figure 3.
Evidence for the existence of brain dysbindin as a stable component of BLOC-1. (a, b) The steady-state protein levels of dysbindin are significantly decreased in brain from BLOC-1-deficient, pallid (pa) mutant mice. “Whole-tissue” detergent extracts were prepared from cerebral cortex, hippocampus and cerebellum dissected from mice at postnatal day (P)45 (a), and from cerebral cortex and hippocampus dissected from P1 mice (b), and then analyzed by immunoblotting using antibodies against the indicated proteins. Besides the major ~50-kDa dysbindin form, minor species of ~42 and ~38 kDa were detected. (c) Reductions in dysbindin protein levels at steady state were also noted in primary hippocampal neurons from pallid mice cultured in Tii medium for 1 or 3 days. (d) Mouse brains were homogenized in the absence of detergents, and the homogenates subjected to ultracentrifugation to obtain a soluble protein fraction and a membrane pellet. Notice that the bulk of dysbindin was recovered from the soluble fraction. (e, f) Soluble protein fractions prepared from P7 brain (e) and adult brain (f) were further fractionated by size-exclusion chromatography, and the resulting fractions were analyzed by immunoblotting using antibodies to dysbindin, pallidin, and a monomeric dysbindin-related protein named CK1BP. The exclusion volume (Vo) and elution positions of proteins with known Stokes’ radii (in Angstroms) are indicated on the top. The elution profile of dysbindin, consisting of a peak corresponding to large Stokes’ radii (95±5 Å, arrowheads), was in close match to that of pallidin (e) and drastically different from that of CK1BP (f).
In the second set of experiments, detergent-free extracts were prepared from wild-type mouse brain, under conditions in which ~90% of dysbindin was recovered in soluble form (Figure 3d), and the cytosolic proteins were then fractionated by high-resolution, size-exclusion chromatography. As shown in Figure 3, panels e and f, the elution profile of dysbindin consisted of a single peak corresponding to a Stokes’ radius of 95±5 Å, a native molecular size which fits within the error range the one previously reported for BLOC-1 from mouse liver (~94 Å).33 Moreover, the elution profile of dysbindin was in close match with that of pallidin (Figure 3e), and there was little or no dysbindin immunoreactivity in fractions corresponding to the size expected for a putative monomeric form, which instead contained the monomeric dysbindin-related protein, CK1BP43 (Stokes’ radius 40±2 Å, Figure 3f).
Taken together, these results suggested that the bulk of dysbindin from brain exists as a stable component of BLOC-1. Consistent with this conclusion, we observed age-dependent declines in the steady-state levels of the pallidin subunit from mouse cerebral cortex, hippocampus and cerebellum that mirrored those observed for the dysbindin protein (Figure 2 and data not shown).
Physical interactions between brain BLOC-1 and SNARE proteins
As part of our attempts to understand the function of dysbindin in brain, we investigated potential interactions with candidate binding partners that had been previously described for isolated BLOC-1 subunits and implicated in neurodevelopment. Specifically, we focused our attention onto two members of the large superfamily of SNARE proteins, SNAP-25 and syntaxin 13. The SNARE proteins are key regulators of docking and fusion of intracellular membranes, including the fusion of vesicles with the plasma membrane during exocytosis.38 They characteristically contain one or two SNARE homology domains of the R, Qa, Qb and Qc types, which can assemble into a tight four-helix bundle containing one copy of each SNARE domain type. It is thought that formation of these SNARE complexes brings two membranes in close proximity and provides the free energy required to drive fusion (see the scheme in Supplementary Figure S2).38 SNAP-25 is best known for its role in synaptic vesicle exocytosis,44 but it also has a demonstrated role in membrane fusion events that occur during the process of neurite outgrowth.45–47 Syntaxin 13 expression in brain is developmentally regulated, and the protein was shown to bind to SNAP-25 and to regulate neurite outgrowth.48 We also focused our attention onto cypin, a guanine deaminidase that regulates dendrite branching.49 Both SNAP-2550 and cypin51 were reported to bind to snapin, while syntaxin 13 was shown to interact with pallidin.42
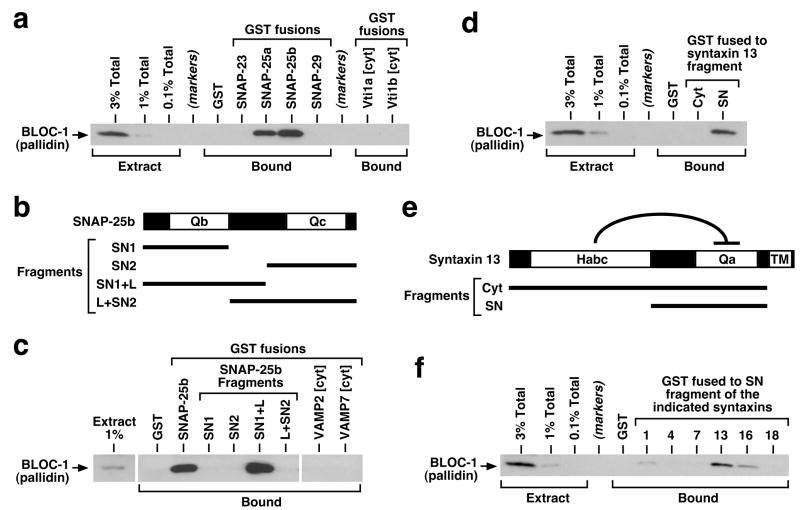
To evaluate the potential significance of these interactions for dysbindin function, we first sought to determine whether any of these proteins could be specifically recognized as a binding partner by the intact BLOC-1 isolated from brain. We used an affinity “pulldown” assay, whereby recombinant forms of the candidate binding partners were immobilized onto beads, and the binding of BLOC-1 from bovine brain cytosol was assessed by immunoblotting using our mAb against the pallidin subunit, which is tightly associated into the complex and virtually undetectable in free form.32,33 As shown in Figure 4a and Supplementary Figure S4, both alternatively spliced variants of SNAP-25 (a and b) robustly interacted with BLOC-1, like SNAP-47 and unlike the related proteins SNAP-23 and -29. Besides SNAP-23,52 other proteins reported to interact with snapin but for which we have failed to detect any interaction with brain BLOC-1 include cypin and regulator of G protein signaling (RGS)7,53 the latter being a paralog of the RGS4 protein encoded by a candidate schizophrenia susceptibility gene54 (Supplementary Figure S4). Specific SNAP-25 binding was observed not only for BLOC-1 from adult bovine brain but also for the native complex from cerebral cortex of P1 and P7 mice (Supplementary Figure S4). Attempts to map the BLOC-1-binding interface using truncated SNAP-25 constructs (Figure 4b) suggested that the Qb domain and part of the linker region connecting it to the Qc domain are both necessary for robust binding to BLOC-1 (Figure 4c). Under the same experimental conditions, the Qb SNARE proteins Vti1a and Vti1b failed to interact with BLOC-1 (Figure 4a), as did the VAMP2 and VAMP7 proteins containing SNARE domains of the R type (Figure 4c). We also observed that a recombinant protein comprising the entire cytoplasmic region of syntaxin 13 was unable to bind brain BLOC-1 (Figure 4d). However, syntaxin 13 and other syntaxins characteristically contain an auto-inhibitory helical region, named Habc, which binds intramolecularly to the Qa SNARE domain (Figure 4e), thus inducing a “closed” conformation.55 We then tested smaller portions of the cytoplasmic region and observed binding of BLOC-1 to a syntaxin 13 fragment, referred herein to as SN, which included the SNARE Qa domain and lacked the Habc domain (Figure 4d). Similar experiments using analogous SN fragments from various syntaxins demonstrated selectivity of BLOC-1 binding towards syntaxin 13 and, to a lesser extent, syntaxins 16 and 1 (Figure 4f).
Figure 4.
Interaction of brain BLOC-1 with recombinant SNARE proteins. (a, c, d, f) Purified GST-fusion proteins were immobilized on glutathione-Sepharose beads and incubated with bovine brain cytosol. Following extensive washing, bound proteins were eluted from beads and analyzed by immunoblotting using a mAb against the pallidin subunit of BLOC-1. Aliquots of the cytosolic extract, corresponding to 0.1–3% of the total amount incubated with each GST-fusion protein, were analyzed in parallel. (a) Both SNAP-25a and -25b interacted robustly with BLOC-1 under conditions in which SNAP-23 and -29, as well as the cytoplasmic regions (cyt) of the Qb SNAREs Vti1a and Vti1b, failed to bind detectable amounts of the complex. (b) Schematic representation of the SNAP-25b protein, which contains the SNARE domains Qb and Qc separated by a linker region. (c) Both the Qb domain and a portion of the linker, but not the Qc domain, were necessary for robust binding of SNAP-25b to BLOC-1. No BLOC-1-binding activity was observed for recombinant proteins bearing the cytoplasmic regions of SNARE proteins of the VAMP family. (e) Schematic representation of the syntaxin 13 protein, which contains an auto-inhibitory Habc domain, a Qa SNARE domain and a transmembrane (TM) region. (d, f) The SN fragment of syntaxin 13, but not the entire cytoplasmic fragment containing the Habc domain, interacted selectively with BLOC-1. SDS-PAGE analyses of all recombinant proteins used in these experiments are shown in Supplementary Figure S3.
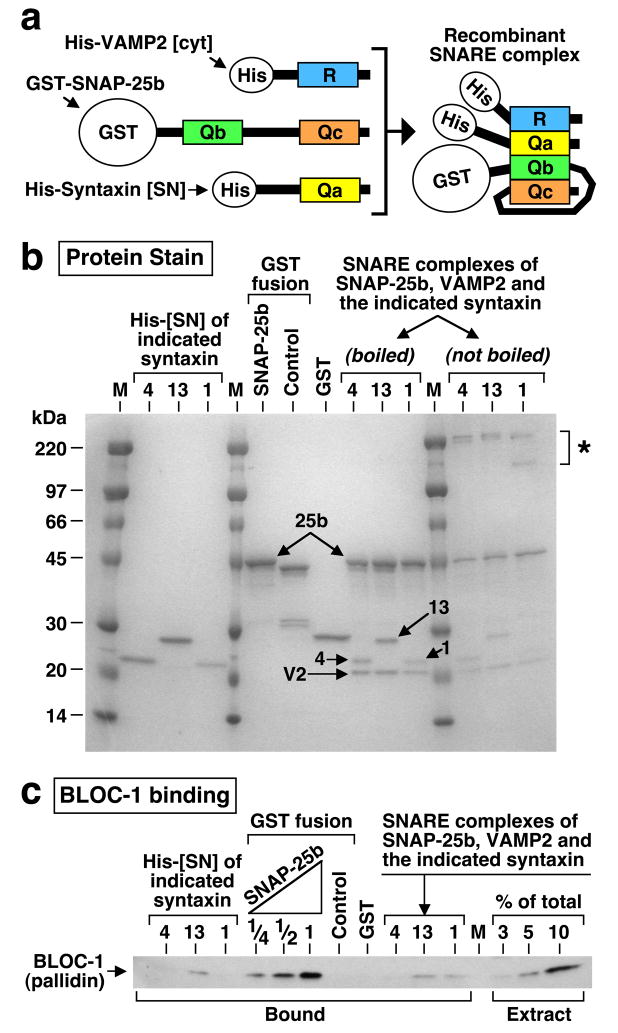
Because SNARE complexes containing SNAP-25, syntaxin 13 and a VAMP protein have been detected in brain,48,56 it was pertinent to ask whether SNARE complex assembly would potentiate, or inhibit, the BLOC-1-binding activity. To this end, purified recombinant proteins comprising full-length SNAP-25, the cytoplasmic portion of VAMP2, and the SN fragment of either syntaxin 1, 4 or 13, were mixed under conditions that allowed formation of recombinant SNARE complexes (Figure 5a), which were then purified from unassembled subunits by two sequential affinity-purification steps. The recombinant complexes were biochemically stable, as judged from the appearance of sodium dodecyl sulfate (SDS)-resistant species38 upon polyacrylamide gel electrophoresis (Figure 5b, asterisk). Next, the abilities of the purified SNARE complexes to bind BLOC-1 from bovine brain cytosol were compared to those of the unassembled subunits. As shown in Figure 5c, there was some detectable binding of BLOC-1 to recombinant complexes containing SNAP-25, VAMP2 and either syntaxin 1 or 13. However, while the extent of BLOC-1 binding to these complexes was comparable to that observed for syntaxin 13 alone, it was significantly less than that observed for an equivalent molar amount of unassembled SNAP-25. Therefore, BLOC-1 displays in vitro a preference towards binding to unassembled SNAP-25, and SNARE complex assembly impairs BLOC-1 binding.
Figure 5.
SNARE complex assembly impairs BLOC-1 binding. (a) Schematic representation of recombinant proteins used to assemble a Qa/Qb/Qc/R SNARE domain complex consisting of full-length SNAP-25b, the cytoplasmic region of VAMP2, and the SNARE domain region of various syntaxins. The GST and His6-S (His) tags are depicted out of scale. (b) SDS-PAGE analysis of recombinant proteins used for the binding assay, including SNARE complexes containing SNAP-25b, the cytoplasmic region of VAMP2 and fragments of syntaxins 1, 4 or 13 (numbered arrows). The amounts of loaded proteins correspond to those used for the binding assay. Notice the presence of slow-migrating species (asterisk) in aliquots of the complexes that were processed for SDS-PAGE without boiling to reveal the existence of SDS-resistant SNARE complexes. (c) The purified SNARE complexes, unassembled SNAP-25b corresponding to 25–100% of the amount incorporated into the complexes, and additional control proteins were immobilized on affinity beads and incubated with bovine brain cytosol. Proteins in the washed beads, as well as aliquots of the cytosol, were analyzed by immunoblotting using a mAb against the pallidin subunit of BLOC-1. Notice that the amounts of BLOC-1 bound to SNARE complexes were significantly less than that bound to unassembled SNAP-25b.
Conflicting results have been reported concerning the impact of dysbindin protein knockdown on SNAP-25 expression levels in cultured cells.24,25 Here, the steady-state levels of SNAP-25, as well as its membrane association and assembly into SDS-resistant SNARE complexes, were examined and found to be normal in cerebral cortex from pallid mice (Supplementary Figure S5), which as mentioned above displays significantly reduced levels of the dysbindin protein (Figure 3a).
Taken together, the above results suggested that brain BLOC-1 is capable of selectively recognizing unassembled SNAP-25 and -47 as well as syntaxin 13 (and other syntaxins) in open conformation.
Neurite extension defects in primary neurons deficient in BLOC-1
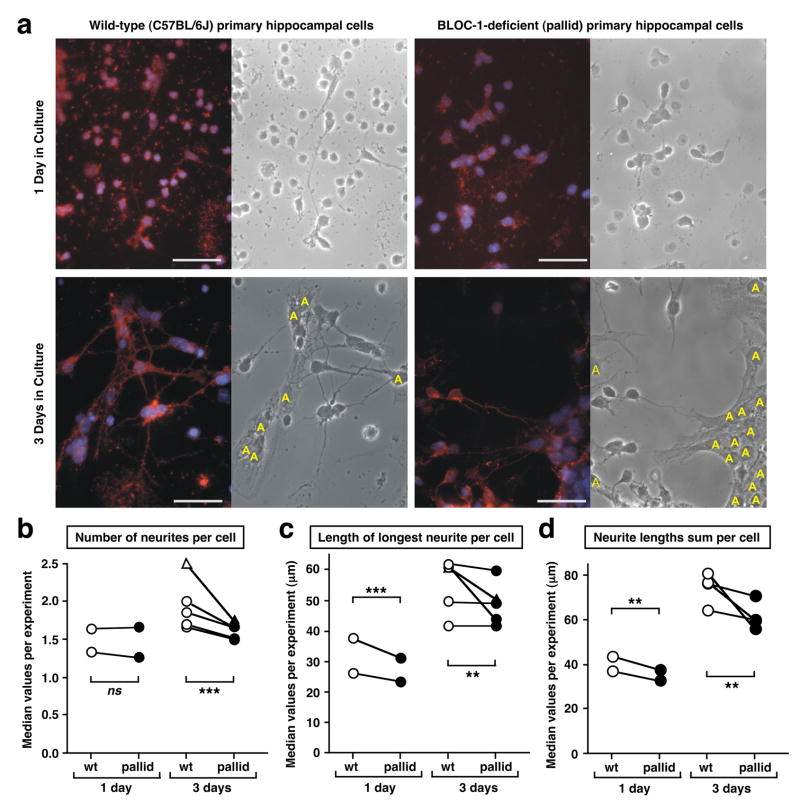
The observed regulation in the levels of the dysbindin protein in brain during development (Figures 1 and 2), together with the in vitro interaction between the dysbindin-containing complex (BLOC-1) and some SNARE proteins (Figures 4 and 5) implicated in neurite outgrowth,45–48,57 prompted us to investigate whether BLOC-1 could play a role in this process. To this end, primary neuronal cultures were established from the hippocampus of newborn (P0-P1) wild-type and BLOC-1-deficient pallid mice. As mentioned above, hippocampal neurons from wild-type mice expressed both pallidin and dysbindin proteins, while those from pallid mice were devoid of pallidin and displayed reduced steady-state levels of the dysbindin protein (Figure 3c). Next, the ability of these cells to extend neurite processes was assessed by morphometric analyses. Representative microscopic images are shown in Figure 6a.
Figure 6.
Absence of BLOC-1 results in neurite outgrowth defects in hippocampal neurons. (a) Representative images of primary hippocampal neurons cultured from wild-type (C57BL/6J) and BLOC-1-deficient (pallid) mice in Tii medium for 1 or 3 days. Cells were fixed in 4% (w/v) paraformaldehyde and stained with antibodies against GAP43 (red). DAPI staining was used to label the cell nuclei (blue). Corresponding phase images are shown. Astrocytes are denoted with “A”. Bars, 50 μm. (b–d) Primary hippocampal neurons were grown in Tii medium (circles) or DMEM medium plus serum (triangles). The number of neurites (b), length of the longest neurite (c) and sum of the lengths of all neurites (d) per neuron were analyzed using a blinded approach. Each pair of symbols connected by a line represents the median values of 15–64 randomly selected images analyzed for wild-type (empty symbols) and pallid mutant (filled symbols) cells in each independent preparation. See Supplementary Table S1 and Figure S6 for further details. Statistical significance was assessed using an ANOVA model as described under Materials and methods. ** P < 0.01, *** P < 0.001, ns, not significant.
Upon one day in culture, hippocampal neurons displayed relatively few neurites (Figure 6a and Supplementary Figure S6) and their number was not significantly affected by BLOC-1 deficiency (P=0.5; Figure 6b). On the other hand, upon three days in culture BLOC-1-deficient neurons extended an average of 14–20% fewer neurites than control neurons (Figure 6b). We then measured neurite lengths, focusing our analysis on the longest neurite per cell (Figure 6c and Supplementary Figure S6); here, the effect of BLOC-1 deficiency was more evident in cells cultured for one day (~15% decrease, P=0.0004) than in those cultured for three days (<10% decrease). We reasoned that these effects could reflect a common underlying phenomenon of compromised membrane delivery to the sites of neurite outgrowth in the absence of BLOC-1, and hence calculated the sum of lengths of all neurites per cell as an indirect estimate of the extent of membrane surface expansion. Interestingly, the effect of BLOC-1 deficiency was consistent upon both one day (~12% decrease, P=0.0043) and three days (~17% decrease, P=0.0027) in culture (Figure 6d), and the overall significance of genotype as an explanatory factor was high for the entire dataset (P<0.0001). Consequently, these results revealed a partial but statistically significant deficit in the ability of BLOC-1-deficient neurons to extend neurites.
Discussion
We have obtained converging evidence for the stable association of brain dysbindin into BLOC-1, and for a potential role of this complex during neurodevelopment. These findings provide a new perspective for considering how allelic variations in the dysbindin-encoding gene could contribute to the risk of developing schizophrenia. Specifically, while our results do not negate proposed mechanisms that involve dysbindin function in the adult brain,22,24,25,27 a role for BLOC-1 in the regulation of neurite outgrowth is reminiscent of those documented for the products of other candidate susceptibility genes, such as DISC1,58–60 and fits within the developmental hypothesis of schizophrenia pathogenesis.29,30
Although the original view of dysbindin as a component of the dystrophin-dystroglycan complex3 had been challenged a few years ago,15,23 the possibilities of dysbindin existing in brain as a monomer, homo-oligomer or assembled into multimeric complexes had not been investigated. Our results imply that the biochemically stable form of brain dysbindin exists as a subunit of BLOC-1. Although the putative existence of a small pool of insoluble dysbindin not assembled into BLOC-1 cannot be completely ruled out, it seems reasonable to infer that BLOC-1 represents the main biologically active form of dysbindin in brain. Such inference provides a conceptual framework for understanding the normal molecular function of dysbindin in brain and how its defects would contribute to the pathogenesis of schizophrenia. Simply put: if the biologically active form of dysbindin is the one assembled into BLOC-1, then it is the entire complex what should be the focus of molecular and functional studies. This view is consistent with a recent report61 of increased schizophrenia risk associated with variations in the gene encoding the BLOS3 subunit, and with an epistatic interaction between those encoding dysbindin and the muted subunit. These genetic variations would contribute to the disease risk by a common mechanism involving reduced BLOC-1 expression. Here, we have pursued this idea by examining protein-protein interactions involving the entire BLOC-1 from brain, and by using a well-established animal model of BLOC-1 deficiency – the pallid – mice for functional studies using primary neuronal cultures.
Although at least 70 candidate-binding partners of individual BLOC-1 subunits have been identified through yeast-two hybrid analyses,62 the vast majority of them has not been tested for their ability to interact with the intact complex. Our finding that the steady-state levels of the complex (as judged from those of dysbindin and pallidin) are developmentally regulated prompted us to focus on a subset of candidate-binding partners that had been implicated in neurodevelopment. We herein report specific in vitro interaction between brain BLOC-1 and two types of SNARE proteins: the Qbc SNAREs SNAP-25 and -47, and the Qa SNARE syntaxin 13 (as well as syntaxins 16 and 1). Because SNARE proteins assemble into heterotypic complexes containing one domain each of the Qa, Qb, Qc and R types,38 it follows that pairs of these BLOC-1-binding partners could associate with each other (and with an R-type SNARE). In fact, a SNARE complex containing both syntaxin 13 and SNAP-25 has been isolated from the developing brain.48 However, our binding analyses indicate that SNARE complex assembly severely impairs BLOC-1 binding, arguing for a potential regulatory role of BLOC-1 during early stages in the SNARE complex assembly process (see model in Supplementary Figure S2).
The observed interactions between BLOC-1 and SNARE proteins provide a plausible mechanism by which BLOC-1 could regulate neurite outgrowth. While the function of SNAP-47 remains obscure,63 other SNAREs that are capable of binding BLOC-1 have been implicated in – among other cellular functions – the delivery of membranes from an intracellular compartment to the growth cone.45–48,57 It is worth mentioning that an endosomal compartment, referred to as the cell outgrowth secretory endosome,64 is considered an important source of intracellular membranes for surface expansion during neurite outgrowth, and that BLOC-1,34 syntaxin 1356,65 and a small pool of SNAP-2547 have been localized to endosomes, at least in certain cell types. A regulatory role for BLOC-1 in the delivery of endosomal membranes to sites of neurite outgrowth would be compatible with the results of our morphometric analyses of BLOC-1-deficient neurons, which displayed the most consistent differences from wild-type neurons when a surrogate of total surface expansion was measured. However, alternative molecular mechanisms cannot be excluded at this point. Besides, the expression of dysbindin in oligodendrocytes and astrocytes (Supplementary Figure S1) raises the possibility that the function of BLOC-1 in brain may not be restricted to that exerted in neurons. Future work will be required to address these points.
Supplementary Material
Acknowledgments
We thank David E. Krantz and Desmond J. Smith for critical reading of the manuscript, and Donna Crandal for help in the preparation of Figure 6. This work was supported in part by National Institutes of Health grant HL068117 and an Independent Investigator Award from NARSAD: The Mental Health Research Association.
Footnotes
Supplementary Information is available at the Molecular Psychiatry website.
References
- 1.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Burmeister M, McInnis MG, Zollner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- 3.Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- 4.Guo AY, Sun J, Riley BP, Thiselton DL, Kendler KS, Zhao Z. The dystrobrevin-binding protein 1 gene: features and networks. Mol Psychiatry. 2009;14:18–29. doi: 10.1038/mp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: Meta-analyses of 12 candidate genes. Schizophr Res. 2008;104:96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutsuddi M, Morris DW, Waggoner SG, Daly MJ, Scolnick EM, Sklar P. Analysis of high-resolution HapMap of DTNBP1 (Dysbindin) suggests no consistency between reported common variant associations and schizophrenia. Am J Hum Genet. 2006;79:903–909. doi: 10.1086/508942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- 10.Kirov G, Ivanov D, Williams NM, Preece A, Nikolov I, Milev R, et al. Strong evidence for association between the dystrobrevin binding protein 1 gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from Bulgaria. Biol Psychiatry. 2004;55:971–975. doi: 10.1016/j.biopsych.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Gornick MC, Addington AM, Sporn A, Gogtay N, Greenstein D, Lenane M, et al. Dysbindin (DTNBP1, 6p22.3) is associated with childhood-onset psychosis and endophenotypes measured by the Premorbid Adjustment Scale (PAS) J Autism Dev Disord. 2005;35:831–838. doi: 10.1007/s10803-005-0028-3. [DOI] [PubMed] [Google Scholar]
- 12.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 13.Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ, et al. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet. 2005;14:1947–1954. doi: 10.1093/hmg/ddi199. [DOI] [PubMed] [Google Scholar]
- 14.Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res. 2008;98:105–110. doi: 10.1016/j.schres.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattori S, Murotani T, Matsuzaki S, Ishizuka T, Kumamoto N, Takeda M, et al. Behavioral abnormalities and dopamine reductions in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Biochem Biophys Res Commun. 2008;373:298–302. doi: 10.1016/j.bbrc.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Feng YQ, Zhou ZY, He X, Wang H, Guo XL, Hao CJ, et al. Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res. 2008;106:218–222. doi: 10.1016/j.schres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Takao K, Toyama K, Nakanishi K, Hattori S, Takamura H, Takeda M, et al. Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Mol Brain. 2008;1:11. doi: 10.1186/1756-6606-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhardwaj SK, Baharnoori M, Sharif-Askari B, Kamath A, Williams S, Srivastava LK. Behavioral characterization of dysbindin-1 deficient sandy mice. Behav Brain Res. 2009;197:435–441. doi: 10.1016/j.bbr.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, Arnold SE. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. doi: 10.1111/j.1601-183X.2009.00477.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazarian R, Starcevic M, Spencer MJ, Dell’Angelica EC. Reinvestigation of the dysbindin subunit of BLOC-1 (biogenesis of lysosome-related organelles complex-1) as a dystrobrevin-binding protein. Biochem J. 2006;395:587–598. doi: 10.1042/BJ20051965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13:2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- 25.Kumamoto N, Matsuzaki S, Inoue K, Hattori T, Shimizu S, Hashimoto R, et al. Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin. Biochem Biophys Res Commun. 2006;345:904–909. doi: 10.1016/j.bbrc.2006.04.163. [DOI] [PubMed] [Google Scholar]
- 26.Talbot K, Cho D-S, Ong W-Y, Benson MA, Han L-Y, Kazi HA, et al. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- 27.Newell-Litwa K, Seong E, Burmeister M, Faundez V. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci. 2007;120:531–541. doi: 10.1242/jcs.03365. [DOI] [PubMed] [Google Scholar]
- 28.Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39:205–216. doi: 10.1016/s0896-6273(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 30.Karlsgodt KH, Sun D, Jimenez AM, Lutkenhoff ES, Willhite R, van Erp TG, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- 31.Falcon-Perez JM, Starcevic M, Gautam R, Dell’Angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet dense granules. J Biol Chem. 2002;277:28191–28199. doi: 10.1074/jbc.M204011200. [DOI] [PubMed] [Google Scholar]
- 32.Moriyama K, Bonifacino JS. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic. 2002;3:666–677. doi: 10.1034/j.1600-0854.2002.30908.x. [DOI] [PubMed] [Google Scholar]
- 33.Starcevic M, Dell’Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J Biol Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- 34.Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SRG, Marks MS, Raposo G, et al. BLOC-1 interacts with BLOC-2 and AP-3 to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 39.Ghiani CA, Ying Z, de Vellis J, Gomez-Pinilla F. Exercise decreases myelin-associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. Glia. 2007;55:966–975. doi: 10.1002/glia.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciciotte SL, Gwynn B, Moriyama K, Huizing M, Gahl WA, Bonifacino JS, et al. Cappuccino, a mouse model of Hermansky-Pudlak syndrome, encodes a novel protein that is part of the pallidin-muted complex (BLOC-1) Blood. 2003;101:4402–4407. doi: 10.1182/blood-2003-01-0020. [DOI] [PubMed] [Google Scholar]
- 41.Gwynn B, Martina JA, Bonifacino JS, Sviderskaya EV, Lamoreux ML, Bennett DC, et al. Reduced pigmentation (rp), a mouse model of Hermansky-Pudlak syndrome, encodes a novel component of the BLOC-1 complex. Blood. 2004;104:3181–3189. doi: 10.1182/blood-2004-04-1538. [DOI] [PubMed] [Google Scholar]
- 42.Huang L, Kuo Y-M, Gitschier J. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nat Genet. 1999;23:329–332. doi: 10.1038/15507. [DOI] [PubMed] [Google Scholar]
- 43.Yin H, Laguna KA, Kuret J. Dysbindin structural homologue CK1BP is an isoform-selective binding partner of human casein kinase-1. Biochemistry. 2006;45:5297–5308. doi: 10.1021/bi052354e. [DOI] [PubMed] [Google Scholar]
- 44.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 45.Osen-Sand A, Catsicas M, Staple JK, Jones KA, Ayala G, Knowles J, et al. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993;364:445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- 46.Delgado-Martinez I, Nehring RB, Sorensen JB. Differential abilities of SNAP-25 homologs to support neuronal function. J Neurosci. 2007;27:9380–9391. doi: 10.1523/JNEUROSCI.5092-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aikawa Y, Lynch KL, Boswell KL, Martin TF. A second SNARE role for exocytic SNAP25 in endosome fusion. Mol Biol Cell. 2006;17:2113–2124. doi: 10.1091/mbc.E06-01-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirling H, Steiner P, Chaperon C, Marsault R, Regazzi R, Catsicas S. Syntaxin 13 is a developmentally regulated SNARE involved in neurite outgrowth and endosomal trafficking. Eur J Neurosci. 2000;12:1913–1923. doi: 10.1046/j.1460-9568.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 49.Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci. 2004;7:145–152. doi: 10.1038/nn1179. [DOI] [PubMed] [Google Scholar]
- 50.Ilardi JM, Mochida S, Sheng ZH. Snapin: a SNARE-associated protein implicated in synaptic transmission. Nat Neurosci. 1999;2:119–124. doi: 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- 51.Chen M, Lucas KG, Akum BF, Balasingam G, Stawicki TM, Provost JM, et al. A novel role for snapin in dendrite patterning: interaction with cypin. Mol Biol Cell. 2005;16:5103–5114. doi: 10.1091/mbc.E05-02-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buxton P, Zhang XM, Walsh B, Sriratana A, Schenberg I, Manickam E, et al. Identification and characterization of Snapin as a ubiquitously expressed SNARE-binding protein that interacts with SNAP23 in non-neuronal cells. Biochem J. 2003;375:433–440. doi: 10.1042/BJ20030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt RA, Edris W, Chanda PK, Nieuwenhuijsen B, Young KH. Snapin interacts with the N-terminus of regulator of G protein signaling 7. Biochem Biophys Res Commun. 2003;303:594–599. doi: 10.1016/s0006-291x(03)00400-5. [DOI] [PubMed] [Google Scholar]
- 54.Levitt P, Ebert P, Mirnics K, Nimgaonkar VL, Lewis DA. Making the case for a candidate vulnerability gene in schizophrenia: Convergent evidence for regulator of G-protein signaling 4 (RGS4) Biol Psychiatry. 2006;60:534–537. doi: 10.1016/j.biopsych.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, et al. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chua CE, Tang BL. Syntaxin 16 is enriched in neuronal dendrites and may have a role in neurite outgrowth. Mol Membr Biol. 2008;25:35–45. doi: 10.1080/09687680701504649. [DOI] [PubMed] [Google Scholar]
- 58.Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, et al. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, et al. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- 60.Bellon A. New genes associated with schizophrenia in neurite formation: a review of cell culture experiments. Mol Psychiatry. 2007;12:620–629. doi: 10.1038/sj.mp.4001985. [DOI] [PubMed] [Google Scholar]
- 61.Morris DW, Murphy K, Kenny N, Purcell SM, McGhee KA, Schwaiger S, et al. Dysbindin (DTNBP1) and the biogenesis of lysosome-related organelles complex 1 (BLOC-1): main and epistatic gene effects are potential contributors to schizophrenia susceptibility. Biol Psychiatry. 2008;63:24–31. doi: 10.1016/j.biopsych.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Fernandez IA, Dell’Angelica EC. A data-mining approach to rank candidate protein-binding partners—The case of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J Inherit Metab Dis. doi: 10.1007/s10545-008-1014-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holt M, Varoqueaux F, Wiederhold K, Takamori S, Urlaub H, Fasshauer D, et al. Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J Biol Chem. 2006;281:17076–17083. doi: 10.1074/jbc.M513838200. [DOI] [PubMed] [Google Scholar]
- 64.Alberts P, Galli T. The cell outgrowth secretory endosome (COSE): a specialized compartment involved in neuronal morphogenesis. Biol Cell. 2003;95:419–424. doi: 10.1016/s0248-4900(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 65.Prekeris R, Foletti DL, Scheller RH. Dynamics of tubulovesicular recycling endosomes in hippocampal neurons. J Neurosci. 1999;19:10324–10337. doi: 10.1523/JNEUROSCI.19-23-10324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.