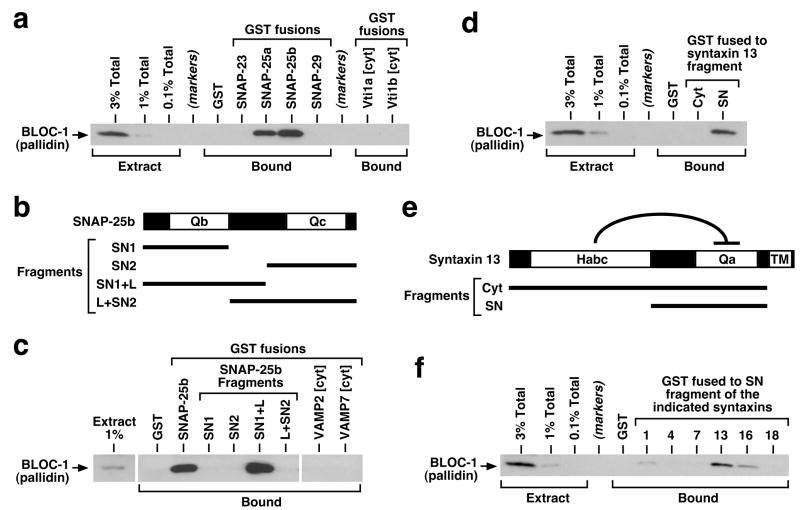
Figure 4.
Interaction of brain BLOC-1 with recombinant SNARE proteins. (a, c, d, f) Purified GST-fusion proteins were immobilized on glutathione-Sepharose beads and incubated with bovine brain cytosol. Following extensive washing, bound proteins were eluted from beads and analyzed by immunoblotting using a mAb against the pallidin subunit of BLOC-1. Aliquots of the cytosolic extract, corresponding to 0.1–3% of the total amount incubated with each GST-fusion protein, were analyzed in parallel. (a) Both SNAP-25a and -25b interacted robustly with BLOC-1 under conditions in which SNAP-23 and -29, as well as the cytoplasmic regions (cyt) of the Qb SNAREs Vti1a and Vti1b, failed to bind detectable amounts of the complex. (b) Schematic representation of the SNAP-25b protein, which contains the SNARE domains Qb and Qc separated by a linker region. (c) Both the Qb domain and a portion of the linker, but not the Qc domain, were necessary for robust binding of SNAP-25b to BLOC-1. No BLOC-1-binding activity was observed for recombinant proteins bearing the cytoplasmic regions of SNARE proteins of the VAMP family. (e) Schematic representation of the syntaxin 13 protein, which contains an auto-inhibitory Habc domain, a Qa SNARE domain and a transmembrane (TM) region. (d, f) The SN fragment of syntaxin 13, but not the entire cytoplasmic fragment containing the Habc domain, interacted selectively with BLOC-1. SDS-PAGE analyses of all recombinant proteins used in these experiments are shown in Supplementary Figure S3.