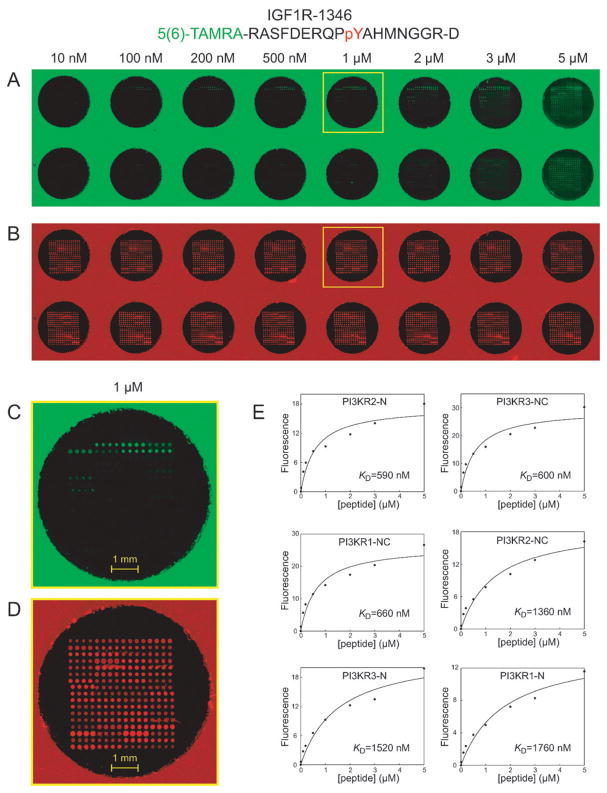
Fig. 2.
Measuring the binding affinity of SH2 and PTB domains for phosphopeptide IGF1R 1346 using protein microarrays. (A) Fluorescence images of SH2 and PTB domain microarrays in separate wells of a 96-well microtiter plate, obtained using a 633 nm laser. The fluorescence arises from a trace amount of Cy5-labeled BSA that was added to each protein before arraying. (B) Fluorescence images of the same SH2 and PTB domain microarrays, probed with eight concentrations of a 5(6)-TAMRA-labeled phosphopeptide derived from IGF1R (pY1346). The images were obtained using a 543 nm laser. (C, D) Close up images of a single well, obtained using 633 nm and 543 nm lasers, respectively. The well corresponds to the one highlighted by a yellow box in panels A and B. (E) Plots showing fluorescence as a function of peptide concentration for six high-affinity interactions. The data were fit to eqn (1) to determine the KD.