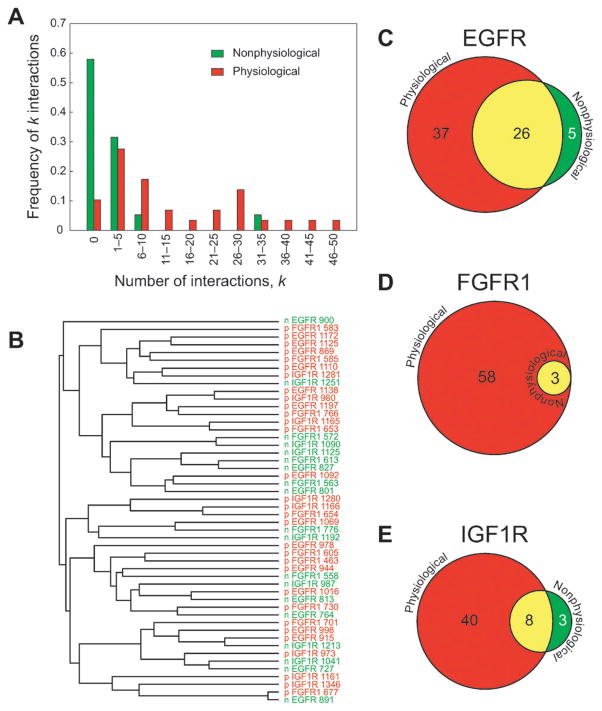
Fig. 4.
Comparison of the physiological and nonphysiological phosphopeptides. (A) Histograms showing the frequency of peptides with different numbers of interactions. Physiological peptides are shown in red; nonphysiological peptides are shown in green. (B) Dendrogram showing phosphopeptides clustered according to their physicochemical properties. Each peptide was described by an 18-dimensional vector comprising the first three z-scales of the three residues up- and downstream of the phosphotyrosine. The vectors were clustered using Euclidean distance as the similarity metric and the dendrogram was prepared using the centroid linkage method. Physiological peptides are designated by a ‘p’ and are colored red; nonphysiological peptides are designated by an ‘n’ and are colored green. (C–E) Venn diagrams showing proteins that interact with phosphopeptides derived from EGFR, FGFR1 and IGF1R, respectively. For each receptor, the circle on the left (red) represents all the proteins with at least one SH2 or PTB domain that interacts with at least one physiological phosphopeptide and the circle on the right (green) represents all the proteins with at least one SH2 or PTB domain that interacts with at least one nonphysiological phosphopeptide. Overlap is colored yellow.