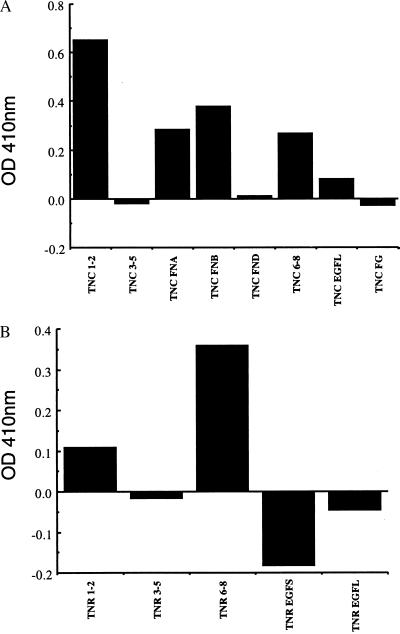
Figure 5.
Binding of GST-β2 to the domains of tenascin-C and tenascin-R. (A) Microtiter plates were coated with 100 nM solutions of the indicated domains of tenascin-C expressed and purified from E. coli. After washing, GST-β2 was added at 40 nM, incubated, washed and quantitated by ELISA as described under Experimental Procedures. The results of a single experiment representative of three separate experiments are illustrated. Binding to TNC FN1–2, TNC FN-A, TNC FN-B, and TNC FN6–8 were consistently greater than background binding in these experiments. (B) A similar experiment was carried out with the indicated domains of tenascin-R.