Abstract
Noggin protein is a potent bone morphogenetic protein (BMP) antagonist capable of inhibiting vasculogenesis even in the presence of provasculogenic VEGF and FGF-2. We found that human umbilical vein endothelial cells (HUVECs) do not express Noggin in culture and used these cells for modeling of antivasculogenesis. We hypothesized that high-efficiency transduction of HUVECs with bicistronic lentiviral vector encoding Noggin and enhanced green fluorescent protein (EGFP) enables direct visualization of Noggin effects in homogenous primary cell populations in vitro and in vivo. By comparing HUVECs transduced with a control GFP and GFP/Noggin expression cassettes, we showed that constitutive and orthotopic Noggin protein expression did not influence cell proliferation, down-regulated BMP-2 expression, and showed no effect on BMP receptor transcripts. We demonstrated that in contrast to GFP-only control, Noggin expression in endothelial cells abrogated endothelial migration in response to monolayer injury, blocked endothelial transmigration, and caused abrogation of cord formation in vitro. Adding exogenous BMP-4 restored the formation of cords. Imaging experiments in vivo investigated vessel formation in Matrigel implants in athymic mice by utilizing GFP imaging or magnetic resonance imaging of perfusion in the implants. Both approaches demonstrated the lack of functional vessel formation after the adoptive transfer of GFP/Noggin-expressing human endothelial cells in mice.—Kang, H.-W., Walvick, R., Bogdanov, A. In vitro and in vivo imaging of antivasculogenesis induced by Noggin protein expression in human venous endothelial cells.
Keywords: HUVEC, BMP, MRI, PGC
Noggin is a glycosylated cysteine-knot chemokine protein (1), which functions as an extracellular negative regulator of transforming growth factor (TGF)β superfamily members (2). Alongside several other antagonists [e.g., crossveinless-2 (BMPR; ref. 3), follistatin, and chordin (4), reviewed in ref. 5], Noggin blocks pluripotent bone morphogenetic protein (BMP) signaling that acts locally on target cells, affecting cell survival, proliferation, and differentiation (6). Noggin inhibits BMPs as a result of forming a neutralizing complex that prevents BMPs from binding to BMP receptors (7). It has been demonstrated that ectopic expression of Noggin in developing embryos results in suppression of lateral somite differentiation and in complete inhibition of chondrogenesis in limbs (8). Recently discovered roles of Noggin in expanding neural stem cells and in promoting dopamine neuron differentiation from human and mouse embryonic stem cells suggest potential applications of Noggin-mediated BMP inhibition in stem cell technology (9, 10). Resent results suggest that human endothelial cell differentiation from pluripotent human embryonic stem cells was inhibited by using Noggin or BMP4-neutralizing antibodies (11).
The regulatory role of Noggin in vascular patterning is also beginning to emerge. For example, as demonstrated recently, BMP-4, which is required for mesoderm formation and vascular/hematopoietic specification, enhances the formation and outgrowth of an immature vascular system (12). By interfering with BMP-4, the exogenously added or ectopically expressed Noggin protein negatively regulates vascular patterning and interferes with endothelial migration (13). Notochord-derived Noggin and Chordin suppress endothelial cell differentiation and maturation processes leading to generation and maintenance of the avascular midline in higher vertebrates (4). Antagonism toward BMP-2 has been also reported as a potential cause of decreased angiogenesis in cancer cell-seeded Matrigel plugs in vivo (14). There are two mechanistic explanations of Noggin-mediated effects in endothelial cells: 1) antiangiogenic effects result from the interruption of BMP-mediated VEGF promoter activation (15); 2) endothelial tubulogenesis and migration inhibition by Noggin are a consequence of down-regulation of E-cadherin via the disruption of β-catenin/Lef1-mediated transcriptional regulation of E-cadherin expression, since Noggin has been reported to induce Lef1-mediated transcription (16).
In the past, the addition of exogenous recombinant Noggin chimeric protein (17) or the ectopic implantation of COS cells secreting Noggin were used to study the effects of Noggin on vasculogenesis in vivo (13). We previously demonstrated that noninvasive imaging can be used for detecting xenotransplanted endothelial cells after adoptive transfer in vivo (18, 19). The goals of the current study were to use transduction of human umbilical vein cells (HUVECs) with bicistronic lentiviral vectors encoding Noggin and imaging marker protein (GFP) to identify the effects of permanent orthotopic Noggin expression and secretion on endothelial proliferation, migration, and ability to form tubular networks; and to enable imaging the effects of Noggin on vasculogenesis in vitro and in vivo.
MATERIALS AND METHODS
Cell culture
HUVECs (passages 1 and 2) were provided by Dr. Bill Luscinskas (Center for Excellence in Endothelial Biology, Brigham and Women’s Hospital, Boston, MA, USA). HUVECs were grown in endothelial cell basal medium (EBM; Lonza, Verviers, Belgium) containing 2% fetal bovine serum and endothelial growth supplements and were used between passages 2 and 5.
Transduction of HUVECs with GFP and bicistronic GFP/Noggin lentiviral vectors
EGFP (enhanced green fluorescent protein) encoded in transfer vector CSCGW2) (20) and bicistronic EGFP/Noggin (CSCW2-hNoggin-IG) lentiviral vectors were kindly provided by Dr. Miguel Sena-Esteves (Department of Neurology, Massachusetts General Hospital, Boston, MA, USA). HUVECs (passage 2) were plated on gelatin-coated T25-flasks, and transduction was performed when cells reached 50% confluence. GFP or bicistronic GFP/Noggin lentiviral vectors were added to the flask at 100 MOI in the presence of polybrene (final concentration 8 μg/ml) and incubated overnight followed by growth medium change. The cells were detached with 0.05% trypsin/EDTA and sorted using GFP expression as a marker. The collected GFP-positive cells were cultured and used for further experiments. Further experiments were carried out with three types of HUVECs, such as wild-type (WT), GFP-expressing (GFP+), and GFP/Noggin-expressing (GFP/Nog+) HUVECs.
Detection of CD31 and Noggin expression
Noggin expression in the HUVECs was confirmed using FACS and immunofluorescence microscopy using rabbit polyclonal anti-Noggin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Before immunolabeling, the cells were permeabilized with 0.5% saponin, 1% horse serum in Dulbecco’s phosphate-buffered saline (DPBS; 4°C, 30 min) followed by fixation in 4% buffered formaldehyde/0.1% glutaraldehyde. PE-conjugated or Cy5.5-conjugated mouse anti-rabbit IgG1 was used as secondary fluorescent antibodies. The detection of CD31 expression was performed by using nonpermeabilized cells incubated with Cy5.5-labeled anti-human CD31 monoclonal antibody (21) (Centocor, Malvern, PA, USA). For FACS analysis, HUVECs were detached by incubating with enzyme-free cell dissociation buffer (Invitrogen, Carlsbad, CA, USA) at 37°C and analyzed using FACScalibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Fluorescence microscopy images were acquired using a Nikon TE2000-U inverted microscope equipped with a 100 W diailluminator and Nikon fluorescence filter cubes (Nikon, Tokyo, Japan). Images were acquired using a CoolSnapHQ-M CCD (Photometrics, Tucson, AZ, USA) and processed using IP Lab Spectrum software (BD Biosciences Bioimaging, Rockville, MD, USA).
Preparation of conditioned medium
EBM (10 ml) was added to a T-75 flask at each of the cell cultures (80% confluent). After 48–72 h, the conditioned medium (CM) was collected and filtered using a 0.22-μm low-protein-binding membrane. The CM was used without further modification in endotube assays. For immunoblotting, the medium was concentrated using centrifugal filters (Amicon Ultra, 10,000 and 50,000 MWCO; Millipore, Billerica, MA, USA) to collect proteins with molecular weights in the range of 10,000–50,000.
Semiquantitative RT-PCR
Total RNA was isolated using TRIzol (Invitrogen) and quantified by using RiboQuant assay (Invitrogen). RT-PCR was performed by using the TitanONE system (Roche Applied Science, Indianapolis, IN, USA). PCR products were analyzed by electrophoresis in 1% agarose gels and visualized by using SYBR Gold staining (Invitrogen) and a UVP BioChemi imaging system (UVP LLC, Upland, CA, USA) equipped with a Hamamatsu CCD (Hamamatsu Photonics, Hamamatsu, Japan). Band intensity was analyzed by using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Noggin primers used were as follows: (F) 5′-TCGAACACCCAGACCCTATC-3′, (R) 5′-TGTAACTTCCTCCGCAGCTT-3′; BMP-2 (F) 5′-AGACCTGTATCGCAGGCACT-3′, (R) 5′-AATTCGGTGATGGAAACTGC-3′; BMP-4 (F) 5′-TGATACCTGAGACGGGGAAG-3′, (R) 5′-ATGTTCTTCGTGGTGGAAGC-3′; BMP receptor (type 1A and 1B, amplification of 100% homology region) (F) 5′-TGGCGAAAAGGTAGCTGTG-3′, (R) 5′-GCAGCAATGAAACCCAAAAT-3′. Human β-actin primers (Roche) were used for housekeeping mRNA amplification and band normalization.
Western blot analysis
Cells were solubilized in 0.1% Igepal, 0.01% SDS solution in 0.1 M Tris, pH 8, in the presence of protease inhibitor mixture (Sigma-Aldrich, St. Louis, MO, USA). Approximately 30 μg of total protein (cell extract or CM concentrate) was loaded onto 4–12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were washed with PBS containing 0.01% Tween 20 (Sigma-Aldrich) and then blocked for nonspecific proteins with 1% nonfat dry milk (Bio-Rad) and 0.5% horse serum in PBS. The membranes were probed with rabbit anti-Noggin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by horseradish peroxidase-labeled goat anti-rabbit antibody (Pierce, Rockford, IL, USA).
In vitro cell proliferation assay
HUVECs (3×104 cells/well) were plated in 24-well plates. Cells were trypsinized and counted at every 12 h (n=6/time point).
In vitro cord formation assay
Four-well cover glass chambers (Lab-Tek; Nunc, Roskilde, Denmark) were coated with Matrigel (5 mg/ml in EBM; Becton Dickinson, Bedford, MA, USA) and allowed to form a gel at 37°C before use. HUVECs (3×104/well) were seeded on the Matrigel surface and incubated at 37°C for 3 h. Tube formation was observed and recorded over the 3- to 24-h period using an inverted microscope. Using the same approach, WT HUVECs were seeded in chambers coated with Matrigel and grown in conditioned medium obtained using cultured WT, GFP+, and GFP/Nog+ cells. In some experiments, hrBMP-4 (Abcam, Cambridge MA, USA) at 20 ng/ml was added to the conditioned medium prior to cell seeding on Matrigel surface. The number of cords per area of gel was determined using four different fields of view in two separate wells (total counts, n=8).
Endothelial cell migration assay
Confluent endothelial cells were wounded by scraping with a 2–200 μl pipette tip, denuding a strip of the monolayer ∼0.3 mm in diameter. The variation in the wound diameter within 3 experiments was ∼5%. Cells were washed twice with Hanks’ solution and incubated with EBM. The rate of wound closure due to cell migration was observed and photographed over a 24-h period.
In vitro Matrigel invasion (transmigration) assay
Matrigel-coated transwell inserts (Costar, Corning, NY, USA; 8-μm filter) were prepared by adding 0.1 ml of Matrigel solution (250 μg/ml) to the transwell and allowing the Matrigel to dry at 37°C in a nonhumidified oven for 24 h.
HUVECs (2×106) were labeled by incubating with 20 μCi of methyl[3H]-thymidine (Perkin-Elmer, Waltham, MA, USA) overnight. The cells were washed with Hanks’ solution 3 times, trypsinized, and then resuspended in low-serum medium (1% serum without growth factors). Cell suspensions (5×104 cells/ml) were then pipetted into transwell filter inserts (transmigration chambers) in 12-well plates containing high-serum medium (5% serum with growth factors) and incubated for 24 h at 37°C in 5% CO2. Migrating and stationary cells were identified as 3 types: nonmigrating cells that remained in the Matrigel, migrating cells that passed through the pores of the filter, and adherent cells on the lower surface of the membrane. The migrating cells were collected in the lower transwell compartment. After washing the filters, nonmigrating cells and the cells in the membrane were collected by wiping the top surface of the filter with a cotton swab and cutting out the filters, respectively. Radioactivity in the 3 fractions was separately determined using a β counter. Migration was expressed as a percentage of cells migrated vs. total cell numbers.
Matrigel implantations in mice
All animal experiments described were approved by the University of Massachusetts Institutional Animal Care and Use Committee in accordance with Federal Regulations for Animal Research. Injections of HUVEC suspensions in growth factor-supplemented Matrigel matrix (BD Sciences, Bedford, MA, USA) were performed as previously described (18, 19). Briefly, female nu/nu mice (NCI) (weight of 20–25 g, n=10) were anesthetized by intraperitoneal injection of a mixture of ketamine (80 mg/kg) and xylazine (12 mg/kg), and flank subcutaneous injections were performed by using a tuberculin syringe with a hypodermic needle (27 gauge). Matrigel mixed with either GFP+ or GFP/Nog+ cells (0.6–0.8 ml) was injected into the right and left posterior flanks of the mice, respectively.
Optical imaging
Mice bearing Matrigel implants containing GFP+ and GFP/Nog+ cells were anesthetized as described above and injected i.v. with 20 μg of Cy5.5-labeled anti-human CD31 monoclonal antibody (22) (Centocor; ∼0.5 nmol of conjugated Cy5.5). The animals were imaged immediately and 3 h postimplantation using Xenogen IVIS100 (XFO-12 fluorescence imaging option, Xenogen, Hopkinton, MA, USA) and Cy5.5 background-corrected excitation filter. Fluorescence was quantitated by using ROI method applied to Live Image (Xenogen)-generated radiance maps.
Magnetic resonance (MR) imaging
Six weeks postinjection of bilateral HUVEC-seeded Matrigel implants, the animals (n=6/group) were anesthetized with 1.5% isoflurane/air and placed in holders equipped with a volume RF coil. A 30-gauge needle catheter connected to polyethylene tubing filled with heparinized saline was placed and secured in the tail vein. The positions of the implants were distinguished by using water (low signal intensity) and Gd (high signal intensity) standards placed next to the implants in the mouse holder. MR images were acquired using a Bruker BioSpin 2.0-T/45-cm inner bore imaging spectrometer equipped with ±20 G/cm self-shielded gradients (Bruker BioSpin Corp., Billerica, MA, USA) or a 3-T Philips Achieva system with a dual (±8 G/cm) gradient system (Philips Healthcare, Andover, MA, USA). A baseline set of fat-suppressed T1-weighted, spin-echo (SE) MR images was obtained at TR/TE = 700/9.2 ms, FOV = 6 × 6 cm, matrix = 256 × 128, NEX = 8, resolution 0.14 × 0.27 × 1.5 mm3. Fat-suppressed gradient-echo (GRE) images were acquired with the same resolution using TR/TE = 29/2.9 ms, flip angle 45°. After acquiring the initial precontrast images, a dose of a long-circulating paramagnetic contrast agent, PGC-Gd (0.05 mmol/kg in 0.1 ml of saline), was slowly injected via the tail vein catheter, and the imaging was repeated within 20 min postinjection using the same pulse sequences. Image sequences (10 slices/animal) were analyzed by using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Regions of interest over the contralateral implants (GFP+ and GFP/Nog+ seeded), muscle, blood vessel (aorta), and area outside the animal (to determine the sd of noise values) were placed before and after the injection of the contrast agent The implant MR signal enhancement ratios were calculated as ER = (SIpost/SDnoise_post)/(SIpre/sdnoise_pre), where SI = mean signal intensity of ROI pixels, and SD = sd of noise measurements. Maximum intensity pixel projection (MIP) images were obtained by using ImageJ software.
Statistical analyses
The calculation of P values in animal experiments was performed by using the Mann-Whitney nonparametric test. In vitro data sets were tested using an unpaired t test with Welch’s correction. Data analysis was performed by using Prism 4 (GraphPad Software, La Jolla CA, USA).
RESULTS
Cell transduction and Noggin expression
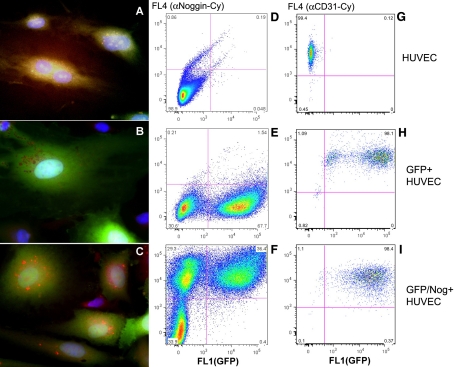
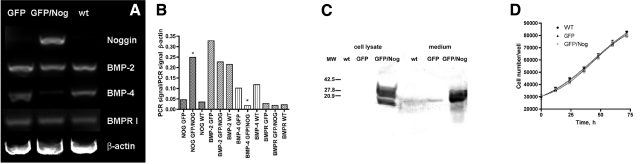
We initially transduced HUVECs with lentiviruses (with a titer 6–8×107 TU/ml). The virus was produced in 293T cells using CSCGW2 transfer vector (20) encoding either a cytomegalovirus minimal promoter-driven expression cassette containing human Noggin and GFP sequences separated by the internal ribosome entry site (IRES), or a cassette containing GFP sequence only (control lentivirus). The expression of GFP and cell transduction efficacy was assessed using fluorescence microscopy. After using the optimized transduction procedure, we found that 90–95% of cells showed expression of GFP. The cells were subjected to preparative sorting to further eliminate nonfluorescent subfraction. The sorted cells were expanded in the culture and analyzed for CD31 expression (Fig. 1) to confirm endothelial phenotype. The expression of Noggin and GFP was analyzed by using saponin-permeabilized cells incubated with polyclonal anti-Noggin antibody followed by either APC-conjugated (Fig. 1A–C) or Cy5.5-conjugated (Fig. 1D–E) secondary antibodies. Fluorescence microscopy showed the presence of red fluorescent (i.e., Noggin-positive) vesicles in the cytoplasm of green fluorescent endothelial cells. These red fluorescent vesicles appeared to contain the major fraction of expressed Noggin protein targeted for secretion. These vesicles were clearly absent in WT or control GFP-positive/Noggin-negative HUVEC (Fig. 1A–C), the latter showing similar nonfluorescent, Noggin-negative compartment, which had cytoplasmic GFP excluded. Flow cytometry showed that only GFP/Nog lentivirus resulted in double-positive HUVECs (i.e., both GFP and Cy dye-positive, ∼36% of total) and that there was a significant loss of fluorescent protein during the saponin permeabilization (Fig. 1B, C) since almost 29% of total cell population showed Noggin expression in the absence of GFP detectable by FACS (Fig. 1F). The presence of intracellular and secreted Noggin in GFP/Nog+-HUVEC-conditioned cell culture medium was further confirmed by immunoblotting (Fig. 2). Cell lysates obtained using wt and GFP+ control HUVEC showed no expression of Noggin in these cells; furthermore, the presence of Noggin in the conditioned cell culture medium was barely detectable (the potential source of Noggin band in control samples is fetal calf serum), whereas both lysates and conditioned medium concentrate obtained using GFP/Nog+ HUVECs clearly showed high levels of intracellular and secretory forms of Noggin protein (Fig. 2). Additional experiments included semiquantitative RT-PCR of total cellular RNA. We observed detectable levels of Noggin mRNA expression only in HUVECs transduced by GFP/Nog+ lentivirus (see Fig. 2A, B). Noggin expression appeared to influence the levels of BMP-2 and BMP-4 mRNA differentially. The normalized levels of BMP-2 mRNA expression were not affected by Noggin overexpression in HUVECs. However, the levels of BMP-4 mRNA expression were markedly decreased in HUVECs transduced with Noggin lentivirus (6–7 times less BMP-4 mRNA normalized to β-actin mRNA levels in GFP/Nog+ HUVECs, P<0.001; Fig. 2B). No changes of BMP type I receptor mRNA levels were observed in either transduced or WT HUVECs.
Figure 1.
Fluorescence microscopy (A–C) and flow cytometry (D–I) of Noggin and GFP expression in lentivirus-transduced WT (A, D, G), GFP+ (B, E, H), and GFP/Nog+ HUVECs (C, F, I). Noggin expression was detected by using rabbit polyclonal anti-Noggin antibody (Santa Cruz Biotechnology, Santa Cruz CA, USA) followed by anti-rabbit-PE conjugate (A–C) or anti-rabbit-Cy5.5 conjugate (D–F). CD31 expression (G–I) was detected by using Cy5.5-labeled monoclonal antibody.
Figure 2.
Analysis of Noggin expression in transduced HUVECs. A) RT-PCR of total RNA using Noggin, BMP-2, BMP-4, BMP receptor (type IA, B) and β-actin specific primers. B) Semiquantitation of results shown in A. C) Immunoblotting of HUVEC lysates and concentrated conditioned media using rabbit polyclonal anti-Noggin antibody followed by anti-rabbit-peroxidase conjugate. D) Comparative growth rates of WT, GFP+, and Nog+/GFP HUVECs. Normalized levels of Nog and BMP-4 mRNA in GFP/Nog+ cells were statistically significantly different from WT and GFP+ cells. *P < 0.001.
HUVEC proliferation, migratory activity, and tube formation
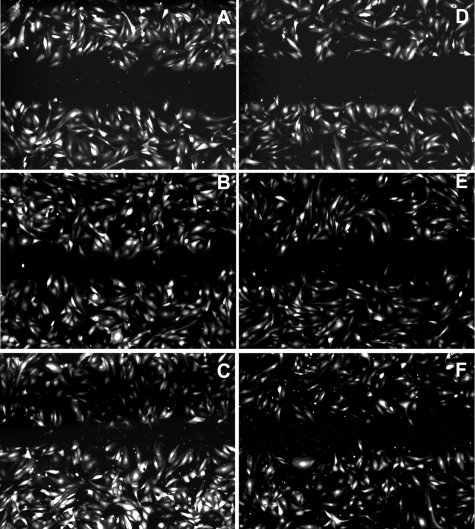
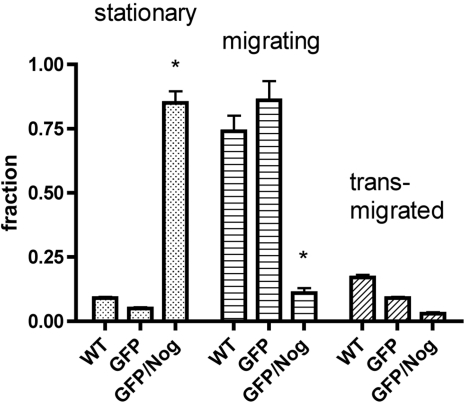
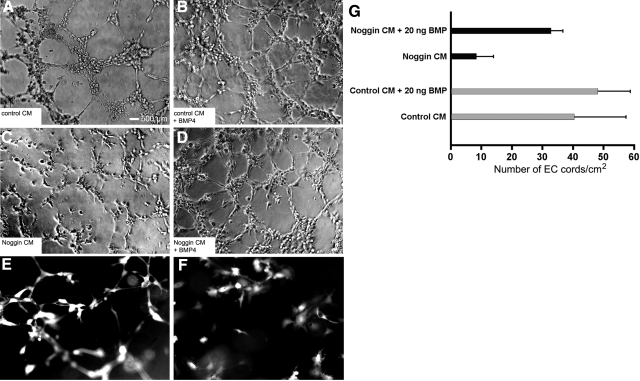
The overexpression of Noggin did not cause any significant effects on HUVEC proliferation. No statistically significant differences in cell numbers in culture measured over the time were detected in either Noggin-overexpressing or control HUVECs; the doubling time was ∼48 h in our experiments (Fig. 2D). The scratch test (monolayer injury test) showed that the response to cell monolayer injury was modified if Noggin was expressed in HUVECs. We observed that GFP+ HUVECs grown on gelatin-covered plastic were migrating beyond the margin of experimental monolayer injury (scratch), with the gap closing by 50% at 6 h after injury, whereas GFP/Nog+ HUVECs had only single cells migrating beyond the margins at that time point (Fig. 3B, E). At 12 h, GFP+ HUVECs continued to show migration, while no apparent decrease of the gap width was observed in the GFP/Nog+ HUVEC culture (Fig. 3C, F). Noggin completely reversed the ability of HUVECs to transmigrate through Matrigel-covered membrane in Transwell inserts (Fig. 4), with >85% nonmigrating, compared to >90% showing migration and transmigration in WT cells (highly statistically significant differences, P<0.001) and somewhat more slowly transmigrating GFP+ HUVECs. However, GFP+ expression alone in control HUVECs did not result in the increase of the number of stationary (nonmigrating) cells (Fig. 4). HUVECs expressing GFP alone were organized into the endotubular cordlike network and were forming intercellular contacts (Fig. 5E). Cell culture medium conditioned by GFP+ HUVECs was neither disrupting nor promoting endotubular networks formed by the WT HUVECs (Fig. 5A). In contrast, GFP/Nog+ HUVECs were forming very few extended and branched cords (Fig. 5F). The cell culture medium obtained after conditioning with GFP/Nog+ HUVECs, which was proven to contain secreted Noggin protein (see above) disrupted the formation of regular cords by WT HUVECs (Fig. 5C) if the medium was used for culturing WT HUVECs on the surface of Matrigel matrix. The addition of BMP4 to the conditioned medium at 20 ng/ml resulted in the increase of cord formation both in the case of GFP/Nog+-HUVEC-conditioned medium and control (GFP+-HUVEC)-conditioned medium (Fig. 5B, D); however, in the latter case, the quantitative differences in the density of cords were less pronounced (Fig. 5G).
Figure 3.
Time-dependent “closure” of injured monolayers of GFP+ (A–C) and GFP/Nog+ HUVECs (D–F). Fluorescent micrographs were taken at 3 h (A, D); 6 h (B, E), and 12 h (C, F) postexperimental injury.
Figure 4.
Fractions of stationary, migrating, and trans-migrated WT, GFP+, and GFP/Nog+ HUVECs determined by using 3H-thymidine-labeled cells placed in Matrigel-coated Transwell membrane inserts. *P < 0.001.
Figure 5.
Effect of Noggin expression on formation of cordlike structures and effect of conditioned cell culture medium on the formation of cords by WT HUVECs. A, C) Conditioned by GFP+ (A) or GFP/Nog+ HUVECs seeded on the surface of Matrigel (C). B, D) Ability of GFP+ (B) and GFP/Nog+ HUVECs (D) to form cords was improved by adding 20 ng/ml hrBMP-4. E, F) Formation of cords by GFP+ (E) and GFP/Nog+ HUVECs (F), detected by using fluorescence microscopy. G) Quantitation of cords using phase-contrast microscopy. Data are presented as means ± sd (n=8). Scale bars= 500 μm.
In vivo effects of Noggin-expressing HUVECs
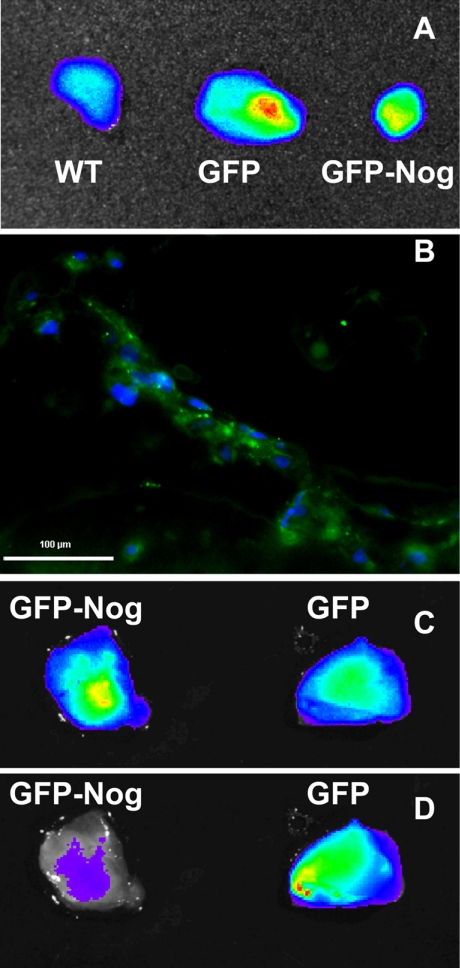
The experiments in vitro suggested that Noggin-overexpressing cells could potentially exhibit modified behavior in vivo after the adoptive transfer in athymic animals. We injected liquid Matrigel seeded with HUVECs in the flanks of nu/nu mice, which led to a rapid solidification and the formation of subcutaneous implants. After 6 wk in vivo, the animals harboring the implants were imaged using a Xenogen camera in fluorescence mode equipped with GFP fluorescence excitation and emission filters. The in vivo imaging did not yield conclusive results due to high background fluorescence in the skin. After the euthanasia and plug dissection, optical imaging was used to determine whether the cells survived and formed vascular networks in vivo. Imaging of Matrigel implants (Fig. 6A) showed that both GFP+ and GFP/Nog+ HUVEC seeding resulted in a higher fluorescence of the implants than in Matrigels seeded with WT cells. However, the volumes of GFP/Nog+-HUVEC-seeded implants were lower than GFP+-HUVEC-seeded ones, and the total fluorescence measured as photon flux from the surface of the latter implants was ∼2.5 times higher than in the case of GFP/Nog+ HUVEC implants. GFP/Nog+ HUVEC implants had the total integrated fluorescence photon fluxes similar to WT HUVEC implants (9.3×107 vs. 8.6×107 photons/s) (Fig. 6A). Only GFP+ HUVEC implants contained vessels lined with fluorescent cells (Fig. 6B). The implants with comparable levels of detectable GFP fluorescence were isolated from the animals preinjected with near-infrared Cy5.5-labeled anti-CD31 antibody. The imaging in GFP and Cy5.5 near-infrared modes showed that GFP/Nog+ HUVEC implants were devoid of near-infrared signal corresponding to the areas of antibody labeling. In contrast, GFP+ HUVEC implants accumulated labeled antibody and showed areas of punctate labeling (Fig. 6C, D).
Figure 6.
A) Fluorescence imaging (photon flux images) of dissected Matrigel implants seeded with transduced and control (WT) HUVECs 6 wk postinjection in the flanks of athymic animals. B) Histology of vessels formed in GFP+-HUVEC-seeded implant. Blue, DAPI; green, GFP. C) Fluorescence imaging of GFP+ HUVEC and GFP/Nog+ HUVEC implants in green channel reflecting GFP+ cell presence. D) Fluorescence imaging of the same implants in the near-infrared (Cy5.5 channel) after injecting animals with anti-CD31-Cy5.5 conjugates.
MR imaging of perfusion in the implants
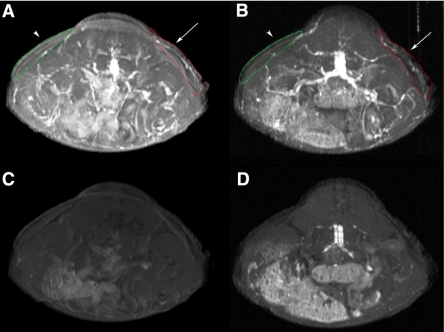
The vascularization of the implants seeded either with WT or with lentivirus-transduced HUVECs was further studied using noninvasive MR imaging. Athymic animals harboring injected Matrigel implants were imaged using MRI before and after intravenous injection of the long-circulating paramagnetic MR contrast agent PGC-Gd. The latter was used for tracking the potential perfusion of tissues with the blood. Our results (Table 1) demonstrate that 6-wk-old implants seeded with WT HUVECs showed markedly higher enhancement ratios if compared to nonseeded contralateral implants (P<0.05) due to the differences in perfusion of the implants with blood. In a group of animals that had contralateral implants seeded with lentivirus-transduced cells, the implants seeded with GFP+ HUVECs had significantly (P<0.05) higher enhancement ratios than those seeded with GFP/Nog+ HUVECs (Table 1). The differences in enhancement ratios of implants either seeded with GFP/Nog+ HUVECs or nonseeded were statistically insignificant (P=0.368). Postcontrast MR images of a representative mouse from the first group (mice 1 to 4) obtained using two different pulse sequences (Fig. 7A, B) demonstrate the visible differences between the implants that were seeded with HUVECs (red trace, arrows) and those that were not (green trace, arrowheads). The precontrast images show few visible differences, though images obtained using GRE pulse sequence due to inherent sensitivity to perfusion show a higher enhancement of HUVEC+ implants (Fig. 7D).
TABLE 1.
Magnetic resonance imaging of Matrigel implants in mice, using a long-circulating contrast agent (PGC-Gd, 0.05 mmol Gd/kg)
| Mouse | Week postimplantation | Implant
|
Enhancement ratio
|
||
|---|---|---|---|---|---|
| Right | Left | Right implant | Left implant | ||
| 1–4; n = 4 | 6 | HUVECs | — | 2.63 ± 0.17* | 1.19 ± 0.21† |
| 5–10; n = 6 | 6 | GFP+ HUVECs | Nog/GFP+ HUVECs | 2.48 ± 0.26* | 1.38 ± 0.32† |
Animals were injected s.c. in the flanks with 0.5 ml Matrigel matrix mixed with cells as indicated (animals 5–10) or plain Matrigel (animals 1–4), and the vessels formed in implants were allowed to mature 6 wk. Enhancement ratio (ER) = (SIpost/SDnoise_post)/(SIpre/SDnoise_pre), where SI = mean signal intensity of ROI pixels and SD = standard deviation of noise ROI measurements; n = 3–4 tomographic slices/mouse (slice thickness, 1.5 mm).
P < 0.05.
P = 0.368 (not significant).
Figure 7.
Representative MR imaging of a mouse implanted with Matrigel seeded with HUVECs as described in Table 1. Maximum intensity pixel projection images obtained using 4 consecutive tomographic axial slices. Images were obtained by using T1-weighted SE pulse sequence (A, C) or GRE pulse sequence (B, D). A, B) Postcontrast images, acquired after injecting PGC-Gd (0.05 μmol Gd/kg i.v.). C, D) Precontrast images. Arrows indicate areas where HUVECs were implanted (red trace). Arrowheads indicate location of control Matrigel implants (green trace).
DISCUSSION
Noggin protein plays a crucial role in regulating signal transduction mediated by BMPs, members of the TGFβ superfamily (22).
There is ample evidence that Noggin is involved in regulation of vasculogenesis and angiogenesis (2, 14). Ectopic implantation of Noggin-secreting cells was shown to induce inhibition of local vascular development (13). Moreover, Noggin coexpression alongside with BMP-4 under the conditions of oscillatory shear stress apparently is involved in controlling proinflammatory signaling in endothelial cells (23).
We set out to investigate the effects of Noggin overexpression directly in HUVECs. We followed cell motility, cord formation, and vasculogenesis in a model system in vivo, which are crucial indicators of the ability of endothelial cells to support angiogenesis in the presence of various cytokines (24, 25). The evidence of a lack of Noggin mRNA and protein expression in human venous endothelial cells suggests differences between HUVEC and cultured human coronary and bovine aortic endothelial cells in the absence of flow. Arterial endothelial cells were positive for mRNA and showed Noggin protein secretion (23). The normalized Noggin mRNA levels and immunoblotting showed that neither WT, nor GFP+ HUVECs expressed Noggin in cell culture conditions (Fig. 2). These results suggested that HUVECs could potentially serve as an excellent model system for investigating Noggin expression effects on vasculogenesis. Lentiviral vector transfer for Noggin cDNA resulted in high levels of secretable Noggin expression in human endothelial cells without any detectable effects on cell proliferation (see Fig. 2D). The latter finding is not surprising, considering that Noggin expression is known to induce antiapoptotic effects in endothelial cells (17). The confirmed expression of Noggin mRNA and/or Noggin protein in HUVECs (Figs. 1 and 2) resulted in strong down-regulation of BMP-4 mRNA but had no effect on BMP-2 mRNA levels (Fig. 2A, B), which potentially could be a consequence of a complete depletion of BMP-4 as a result of trapping by overexpressed Noggin with a resultant negative feedback suppression of BMP-4 gene expression. As we expected, Noggin expression showed typical strong antimotility effects in endothelial cells, resulting in an inefficient model monolayer injury closure in endothelial monolayer and an 85% inhibition of cell transmigration through Matrigel-coated inserts (Figs. 3 and 4). The ability of GFP/Nog+-HUVEC-conditioned medium to cause dramatic inhibition of the ability of WT HUVECs to form branched cordlike networks prompted us to investigate the potential Noggin-mediated effects on endothelial cell survival and vessel formation after the adoptive transfer in vivo (18, 26). We previously used injectable mixtures of cells and Matrigel matrix for studying human endothelial cell-lined immature vessel formation and stabilization in athymic animals using noninvasive MR and optical imaging modalities (18, 19). The above imaging approach allowed estimation of the presence of endothelial cells in the matrix by using specific, endothelium-targeted contrast agents (27). In the current study, we expected that imaging of implants could be assisted by the endogenous expression of GFP in endothelial cells, enabling fluorescence detection in live mice. However, GFP expression was unequivocal only if observed ex vivo in dissected Matrigel implants (Fig. 6). Only the implants containing control GFP+cells showed high fluorescence that was due to the presence of GFP+ HUVECs lining the vessels. The imaging in the near-infrared range with indocyanine -labeled anti-human PECAM-1 (CD31) antibody additionally confirmed that the GFP+ HUVECs formed perfused and endothelial-lined vessels (Fig. 6). Finally, by using MR imaging, we succeeded in comparing GFP+ and GFP/Nog+ HUVECs in contralateral implants by using long-circulating MR contrast agent as a marker of blood pool in live animals (28, 29). PGC-assisted MRI allowed the detection of changes in MR signal intensity due to perfusion of tissues with blood (Fig. 7). These experiments additionally confirmed that HUVEC- or GFP+-HUVEC-seeded implants had higher MR SE ratios than control implants (Table 1). The lack of perfusion in these implants is a consequence of two potential effects of Noggin secretion: disruption of HUVEC migration and tubulogenesis; and inhibition of mouse blood vessel ingrowth into the implants, which is essential for establishing circulation in chimeric mouse-human blood vessels (19, 30). Since Matrigel was supplemented with high amounts of both FGF-2 and VEGF before implantation, we concluded that strong Noggin-mediated BMP antagonism rather than VEGF down-regulation was primarily responsible for the initial disruption of intercellular contacts leading to the lacking perfused vessel-like structures in the case of GFP/Nog+ HUVECs.
Overall, our results support the hypothesis that the constitutive expression of BMP-antagonizing Noggin protein in endothelial cells results in the efficient disruption of intercellular contacts, as well as cell motility, which translates in the abrogation of vessel formation on adoptive transfer of these cells into athymic animals. The observed effects suggest that the induction of Noggin expression (or supplementation with Noggin) is potentially efficient in reversing angiogenesis and proinflammatory changes in the repertoire of endothelial adhesion molecules that are induced by altered sheared stress (31).
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grant RO1-EB000858 (A.B.). The authors are grateful to Dr. Miguel Sena-Esteves (Department of Neurology, Massachusetts General Hospital, Boston, MA, USA) for providing high-titer lentiviral vectors, Dr. Marian Nakada (Centocor, Malvern, PA, USA) for supplying anti-human CD31 monoclonal antibody, and Bill Luscinskas (Brigham and Women’s Hospital, Boston, MA, USA) for providing HUVECs.
References
- Smith W C, Harland R M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Nimmagadda S, Geetha Loganathan P, Huang R, Scaal M, Schmidt C, Christ B. BMP4 and noggin control embryonic blood vessel formation by antagonistic regulation of VEGFR-2 (Quek1) expression. Dev Biol. 2005;280:100–110. doi: 10.1016/j.ydbio.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch V L, Conlon F L, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan M, Davis P, Timmer J, Herzlinger D, Mikawa T. Notochord-derived BMP antagonists inhibit endothelial cell generation and network formation. Dev Biol. 2009;326:101–111. doi: 10.1016/j.ydbio.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides A N, Kwiatkowski W, Affolter M, Vale W W, Belmonte J C, Choe S. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Johnson R L. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998;197:205–217. doi: 10.1006/dbio.1997.8824. [DOI] [PubMed] [Google Scholar]
- Bonaguidi M A, Peng C Y, McGuire T, Falciglia G, Gobeske K T, Czeisler C, Kessler J A. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28:9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Lee Y M, Zhou W, Freed C R. Noggin enhances dopamine neuron production from human embryonic stem cells and improves behavioral outcome after transplantation into Parkinsonian rats. Stem Cells. 2008;26:2810–2820. doi: 10.1634/stemcells.2008-0085. [DOI] [PubMed] [Google Scholar]
- Kelly M A, Hirschi K K. Signaling hierarchy regulating human endothelial cell development. Arterioscler Thromb Vasc Biol. 2009;29:718–724. doi: 10.1161/ATVBAHA.109.184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd N L, Dhara S K, Rekaya R, Godbey E A, Hasneen K, Rao R R, West F D, 3rd, Gerwe B A, Stice S L. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med. 2007;232:833–843. [PubMed] [Google Scholar]
- Reese D E, Hall C E, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- Langenfeld E M, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- Dai J, Kitagawa Y, Zhang J, Yao Z, Mizokami A, Cheng S, Nor J, McCauley L K, Taichman R S, Keller E T. Vascular endothelial growth factor contributes to the prostate cancer-induced osteoblast differentiation mediated by bone morphogenetic protein. Cancer Res. 2004;64:994–999. doi: 10.1158/0008-5472.can-03-1382. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono M, Shibuya M. Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression. Mol Cell Biol. 2003;23:4627–4636. doi: 10.1128/MCB.23.13.4627-4636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H W, Torres D, Wald L, Weissleder R, Bogdanov A A., Jr Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006;86:599–609. doi: 10.1038/labinvest.3700421. [DOI] [PubMed] [Google Scholar]
- Bogdanov A A, Jr, Lin C P, Kang H W. Optical imaging of the adoptive transfer of human endothelial cells in mice using anti-human CD31 monoclonal antibody. Pharm Res. 2007;24:1186–1192. doi: 10.1007/s11095-006-9219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena-Esteves M, Tebbets J C, Steffens S, Crombleholme T, Flake A W. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Nakada M T, Amin K, Christofidou-Solomidou M, O'Brien C D, Sun J, Gurubhagavatula I, Heavner G A, Taylor A H, Paddock C, Sun Q H, Zehnder J L, Newman P J, Albelda S M, DeLisser H M. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J Immunol. 2000;164:452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Chang K, Weiss D, Suo J, Vega J D, Giddens D, Taylor W R, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- Hotfilder M, Nowak-Gottl U, Wolff J E. Tumorangiogenesis: a network of cytokines. Klin Padiatr. 1997;209:265–270. doi: 10.1055/s-2008-1043960. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- Skovseth D K, Yamanaka T, Brandtzaeg P, Butcher E C, Haraldsen G. Vascular morphogenesis and differentiation after adoptive transfer of human endothelial cells to immunodeficient mice. Am J Pathol. 2002;160:1629–1637. doi: 10.1016/S0002-9440(10)61110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H W, Josephson L, Petrovsky A, Weissleder R, Bogdanov A., Jr Magnetic resonance imaging of inducible E-selectin expression in human endothelial cell culture. Bioconj Chem. 2002;13:122–127. doi: 10.1021/bc0155521. [DOI] [PubMed] [Google Scholar]
- Lewin M, Bredow S, Sergeyev N, Marecos E, Bogdanov A, Jr, Weissleder R. In vivo assessment of vascular endothelial growth factor-induced angiogenesis. Int J Cancer. 1999;83:798–802. doi: 10.1002/(sici)1097-0215(19991210)83:6<798::aid-ijc16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kim Y R, Yudina A, Figueiredo J, Reichardt W, Hu-Lowe D, Petrovsky A, Kang H W, Torres D, Mahmood U, Weissleder R, Bogdanov A A., Jr Detection of early antiangiogenic effects in human colon adenocarcinoma xenografts: in vivo changes of tumor blood volume in response to experimental VEGFR tyrosine kinase inhibitor. Cancer Res. 2005;65:9253–9260. doi: 10.1158/0008-5472.CAN-03-2619. [DOI] [PubMed] [Google Scholar]
- Koike N, Fukumura D, Gralla O, Au P, Schechner J S, Jain R K. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan A P. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254–260. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]