Abstract
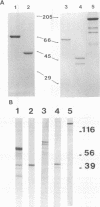
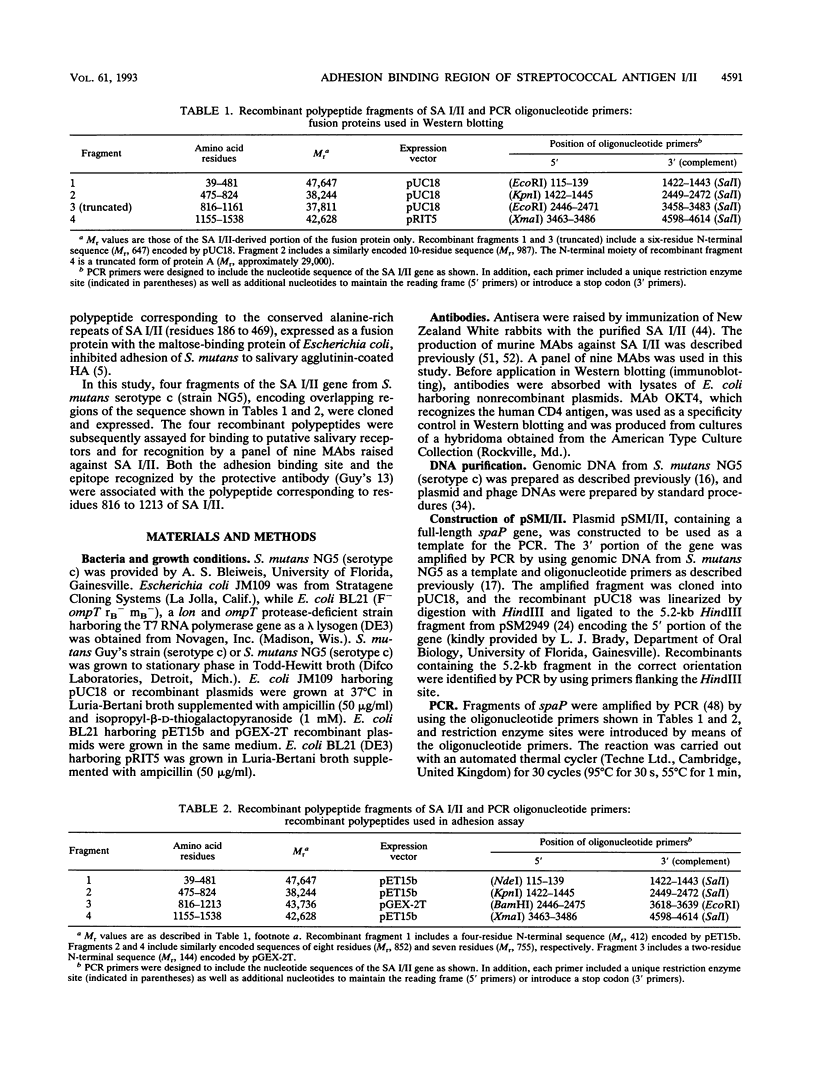
Attachment of Streptococcus mutans to the tooth surface involves a cell surface protein with an M(r) of 185,000, termed streptococcal antigen (SA) I/II. Four overlapping fragments of the gene encoding SA I/II were amplified by polymerase chain reaction, cloned, and expressed in Escherichia coli. The recombinant polypeptides were assayed for adhesion-binding activity to salivary receptors and for recognition by a panel of monoclonal antibodies (MAbs) raised against SA I/II. Two of the MAbs which are known to prevent colonization of S. mutans in vivo bound the recombinant polypeptide comprising residues 816 to 1161. In vitro adhesion of S. mutans to saliva-coated hydroxyapatite beads was also inhibited specifically by a polypeptide (residues 816 to 1213) encompassing the same region. The evidence from the MAbs preventing colonization of S. mutans and the adherence inhibition assay suggests that an adhesion-binding activity resides within the portion of SA I/II comprising residues 816 to 1213, which is highly conserved among oral streptococcal species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature. 1989 Feb 23;337(6209):709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- Brady L. J., Piacentini D. A., Crowley P. J., Oyston P. C., Bleiweis A. S. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect Immun. 1992 Mar;60(3):1008–1017. doi: 10.1128/iai.60.3.1008-1017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian R., Schulz G., Schuster-Kolbe J., Allmaier G., Schmid E. R., Sleytr U. B., Messner P. Complete structure of the tyrosine-linked saccharide moiety from the surface layer glycoprotein of Clostridium thermohydrosulfuricum S102-70. J Bacteriol. 1993 Mar;175(5):1250–1256. doi: 10.1128/jb.175.5.1250-1256.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley P. J., Brady L. J., Piacentini D. A., Bleiweis A. S. Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect Immun. 1993 Apr;61(4):1547–1552. doi: 10.1128/iai.61.4.1547-1552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Berthold P., Leboy P. S., Golub E. E., Davis C. A., Malamud D. Saliva-mediated aggregation of Enterococcus faecalis transformed with a Streptococcus sanguis gene encoding the SSP-5 surface antigen. Infect Immun. 1989 May;57(5):1470–1475. doi: 10.1128/iai.57.5.1470-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Davis C. A., Corner A. M., Lamont R. J., Leboy P. S., Malamud D. Cloning and expression of a Streptococcus sanguis surface antigen that interacts with a human salivary agglutinin. Infect Immun. 1988 Sep;56(9):2484–2490. doi: 10.1128/iai.56.9.2484-2490.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- Demuth D. R., Lammey M. S., Huck M., Lally E. T., Malamud D. Comparison of Streptococcus mutans and Streptococcus sanguis receptors for human salivary agglutinin. Microb Pathog. 1990 Sep;9(3):199–211. doi: 10.1016/0882-4010(90)90022-i. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res. 1989 Sep;68(9):1303–1307. doi: 10.1177/00220345890680090201. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. M., Thoren-Gordon M., Curtiss R., 3rd Regions of the Streptococcus sobrinus spaA gene encoding major determinants of antigen I. J Bacteriol. 1990 Jul;172(7):3988–4001. doi: 10.1128/jb.172.7.3988-4001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G., Nikolova E., Russell M. W. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun. 1992 Dec;60(12):5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Smorawinska M., Curtiss R., 3rd Expression of Streptococcus mutans aspartate-semialdehyde dehydrogenase gene cloned into plasmid pBR322. J Gen Microbiol. 1982 May;128(5):1135–1145. doi: 10.1099/00221287-128-5-1135. [DOI] [PubMed] [Google Scholar]
- Kelly C., Evans P., Bergmeier L., Lee S. F., Progulske-Fox A., Harris A. C., Aitken A., Bleiweis A. S., Lehner T. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 1989 Nov 20;258(1):127–132. doi: 10.1016/0014-5793(89)81632-1. [DOI] [PubMed] [Google Scholar]
- Kishimoto E., Hay D. I., Gibbons R. J. A human salivary protein which promotes adhesion of Streptococcus mutans serotype c strains to hydroxyapatite. Infect Immun. 1989 Dec;57(12):3702–3707. doi: 10.1128/iai.57.12.3702-3707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto E., Hay D. I., Gibbons R. J. Inhibition of adhesion-promoting activity of a human salivary protein which promotes adhesion of Streptococcus mutans JBP to hydroxyapatite. FEMS Microbiol Lett. 1991 Dec 15;69(1):19–22. doi: 10.1016/0378-1097(91)90639-r. [DOI] [PubMed] [Google Scholar]
- Koga T., Okahashi N., Takahashi I., Kanamoto T., Asakawa H., Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990 Feb;58(2):289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPolla R. J., Haron J. A., Kelly C. G., Taylor W. R., Bohart C., Hendricks M., Pyati J. P., Graff R. T., Ma J. K., Lehner T. Sequence and structural analysis of surface protein antigen I/II (SpaA) of Streptococcus sobrinus. Infect Immun. 1991 Aug;59(8):2677–2685. doi: 10.1128/iai.59.8.2677-2685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Bleiweis A. S. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988 Aug;56(8):2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Erdos G. W., Piacentini D. A., Ayakawa G. Y., Crowley P. J., Bleiweis A. S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect Immun. 1989 Nov;57(11):3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Caldwell J., Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985 Dec;50(3):796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Rowan J., Straffon L. H., Loos P. J. Association of Streptococcus mutants with human dental decay. Infect Immun. 1975 Jun;11(6):1252–1260. doi: 10.1128/iai.11.6.1252-1260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwenadler B., Nilsson B., Abrahmsén L., Moks T., Ljungqvist L., Holmgren E., Paleus S., Josephson S., Philipson L., Uhlén M. Production of specific antibodies against protein A fusion proteins. EMBO J. 1986 Sep;5(9):2393–2398. doi: 10.1002/j.1460-2075.1986.tb04509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Hunjan M., Smith R., Kelly C., Lehner T. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect Immun. 1990 Oct;58(10):3407–3414. doi: 10.1128/iai.58.10.3407-3414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Hunjan M., Smith R., Lehner T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin Exp Immunol. 1989 Sep;77(3):331–337. [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Kelly C. G., Munro G., Whiley R. A., Lehner T. Conservation of the gene encoding streptococcal antigen I/II in oral streptococci. Infect Immun. 1991 Aug;59(8):2686–2694. doi: 10.1128/iai.59.8.2686-2694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Smith R., Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987 May;55(5):1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. G., Smith R., Lehner T. Recognition of carbohydrate and protein epitopes by monoclonal antibodies to a cell wall antigen from Streptococcus mutans. Infect Immun. 1987 Mar;55(3):810–815. doi: 10.1128/iai.55.3.810-815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier J. A., Scholler M., Leproivre Y., Pini A., Sommer P., Klein J. P. Complete nucleotide sequence of the sr gene from Streptococcus mutans OMZ 175. FEMS Microbiol Lett. 1990 Mar 1;56(1-2):223–227. doi: 10.1016/0378-1097(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol. 1989 May;3(5):673–678. doi: 10.1111/j.1365-2958.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Takahashi I., Nakai M., Senpuku H., Nisizawa T., Koga T. Identification of antigenic epitopes in an alanine-rich repeating region of a surface protein antigen of Streptococcus mutants. Infect Immun. 1993 Apr;61(4):1301–1306. doi: 10.1128/iai.61.4.1301-1306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. Its implication for the attachment of surface proteins in gram-positive bacteria. J Exp Med. 1989 Dec 1;170(6):2119–2133. doi: 10.1084/jem.170.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Mansson-Rahemtulla B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol Immunol. 1989 Jun;4(2):106–111. doi: 10.1111/j.1399-302x.1989.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Davern K. M., Board P. G., Tiu W. U., Garcia E. G., Mitchell G. F. Mr 26,000 antigen of Schistosoma japonicum recognized by resistant WEHI 129/J mice is a parasite glutathione S-transferase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8703–8707. doi: 10.1073/pnas.83.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Lehner T., Beverley P. C. Characterization of monoclonal antibodies to Streptococcus mutans antigenic determinants I/II, I, II, and III and their serotype specificities. Infect Immun. 1984 Oct;46(1):168–175. doi: 10.1128/iai.46.1.168-175.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R., Lehner T. Characterisation of monoclonal antibodies to common protein epitopes on the cell surface of Streptococcus mutans and Streptococcus sobrinus. Oral Microbiol Immunol. 1989 Sep;4(3):153–158. doi: 10.1111/j.1399-302x.1989.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Staffileno L. K., Hendricks M., LaPolla R., Bohart C., Van Hook P., Rosen J. I., Warner J., Hoey K., Wegemer D., Naso R. B. Cloning of the amino terminal nucleotides of the antigen I/II of Streptococcus sobrinus and the immune responses to the corresponding synthetic peptides. Arch Oral Biol. 1990;35 (Suppl):47S–52S. doi: 10.1016/0003-9969(90)90130-3. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Matsushita K., Nisizawa T., Okahashi N., Russell M. W., Suzuki Y., Munekata E., Koga T. Genetic control of immune responses in mice to synthetic peptides of a Streptococcus mutans surface protein antigen. Infect Immun. 1992 Feb;60(2):623–629. doi: 10.1128/iai.60.2.623-629.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Matsushita K., Tokuda M., Kanamoto T., Munekata E., Russell M. W., Koga T. Immunogenicity and protective effect against oral colonization by Streptococcus mutans of synthetic peptides of a streptococcal surface protein antigen. J Immunol. 1991 Jan 1;146(1):332–336. [PubMed] [Google Scholar]
- Tokuda M., Okahashi N., Takahashi I., Nakai M., Nagaoka S., Kawagoe M., Koga T. Complete nucleotide sequence of the gene for a surface protein antigen of Streptococcus sobrinus. Infect Immun. 1991 Sep;59(9):3309–3312. doi: 10.1128/iai.59.9.3309-3312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]