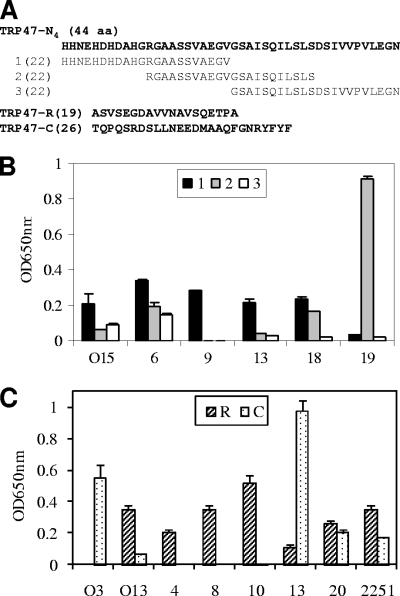
FIG. 5.
Immunoreactivities of overlapping synthetic peptides spanning E. chaffeensis TRP47-N4 and the synthetic TRP47-R and TRP47-C peptides, as determined by ELISA. (A) Sequences of three overlapping peptides spanning the TRP47-N4 fragment and the TRP47-R and TRP47-C peptides. (B) TRP47-N4 peptides reacted with five HME patient serum samples (no. O15, 6, 9, 13, 18, and 19) by ELISA. (C) The TRP47-R and TRP47-C peptides reacted with seven HME patient serum samples (no. O3, O13, 4, 8, 10, 13, and 20) and an anti-E. chaffeensis dog serum sample (no. 2251) by ELISA. The OD readings represent the mean values for three wells (± standard deviations), with the OD values of the buffer-only wells subtracted. The OD readings of peptide TRP47-R were significantly (P < 0.05) higher than those of TRP47-C for all patient sera except for sample no. O3 and 13, for which the OD readings of peptide TRP47-C were significantly (P < 0.05) higher than those of TRP47-R. The normal human or dog sera did not recognize TRP47 polypeptides (data not shown).