Abstract
To investigate striatal mechanisms underlying the acute effects of stimulants on motor behavior, firing rates (FRs) of striatal neurons related specifically to vertical head movement were studied exclusively during vertical head movements. Precocaine FRs were recorded after intraperitoneal saline injection (time 1; T1), and rats performed conditioned vertical head movements (>10,000) similar to those induced by stimulants. After cocaine injection (0, 5, 10, or 20 mg/kg; T2), animals continued in the task. The proportion of long head movements was increased by low doses but decreased by the high dose, which induced stereotypic head movements. Comparing each neuron's FR during movements that were matched between T1 and T2 (e.g., regarding direction, distance), cocaine's effects depended on predrug FR and dose. Plots regressing T2FR on T1FR showed dose-dependent, “clockwise” rotations of regression lines in plots of all the neurons' average FRs and of individual neurons' FRs during different movements. All three doses elevated normally low FRs; the high dose also suppressed many higher FRs. Enhancement of a neuron's FR associated with weak and suppression of FR associated with strong corticostriatal inputs contradict several current theories of dopamine (DA) function. Induction of stereotypy by a single, high-dose injection suggests that this cocaine level exceeded that in other studies using cocaine self-administration, in which stereotypy develops only after several sessions. Suppressive effects observed only at the high dose and in numerous electrophysiological studies of DA agonist effects may be unrepresentative of uniform elevations in lateral striatal firing related to movement observed at lower cocaine levels.
Effects of psychostimulants include increased rates of grooming, social activity, rearing, and locomotion at low doses; increased rates of locomotion and rearing at the expense of feeding, grooming, and social activity at moderate doses; whereas complex movements are replaced by short, repetitive, apparently purposeless movements, termed stereotypy, such as head bobbing at high doses (Lyon and Robbins, 1975). Dopamine (DA) transmission in the lateral striatum plays a key role in psychostimulant-induced stereotypy (Creese and Iversen, 1974; Kelly et al., 1975), more so than non-DA monoamines (Fog and Pakkenberg, 1971; Creese and Iversen, 1974). DA agonists in the lateral, but not medial, striatum cause stereotypy (Kelley and Delfs, 1994). DA antagonists or 6-hydroxydopamine lesions of striatal DA attenuate psychostimulant-induced stereotypy (Kelly et al., 1975).
Glutamatergic projections from somatic sensorimotor cortices converge in dorsolateral striatum (DLS) (Künzle, 1975; McGeorge and Faull, 1989). Striatal medium spiny neurons (MSNs) fire in relation to sensorimotor activity of particular body parts (Crutcher and DeLong, 1984; Carelli and West, 1991) and project via pallidum and substantia nigra pars reticulata to thalamocortical premotor areas (Parent and Hazrati, 1995). The convergence onto MSNs of glutamatergic inputs and DAergic input from the substantia nigra pars compacta (Freund et al., 1984; Parent and Hazrati, 1995) is essential for psychostimulant-induced stereotypy (Kelley and Delfs, 1994), as well as for normal motor function.
Despite evidence implicating the striatum in psychostimulant-induced movements, and despite the replicable observation that the only direction of change in the activity of DLS neurons during movement is to increase firing rate (FR), effects of systemic stimulants on FR of DLS neurons during those movements remain poorly understood because of mixed previous results, including inhibition of some neurons and excitation of others (e.g., Trulson and Jacobs, 1979; Haracz et al., 1998). After stimulant administration, pharmacological and behavioral variables both change, potentially obscuring whether changes in firing are attributable to either one or to both. Some studies may not have precisely accounted for correlations of firing with sensorimotor variables, possibly distorting analyses of FR changes coincident with psychostimulant-induced motoric changes and their associated alterations in sensory feedback to striatum.
Therefore, we assessed cocaine's effects on striatal FR during movements that occur similarly before and after injection (Ellinwood and Kilbey, 1975; Lyon and Robbins, 1975). The method anticipates cocaine-induced movements, such as head bobbing or oral behaviors, so that comparisons of firing between cocaine and precocaine periods eliminate differences in sensorimotor behavior. We reinforced vertical head movements and selected only DLS neurons correlated specifically with vertical head movement (note that studying other neuronal types requires matching of the movements to which they are related) (Tang et al., 2008). Conditioning the correlated movement enabled the collection of thousands of different head movements, and thus numerous independent observations of each neuron's FR under behavioral and environmental conditions that were similar in both precocaine and cocaine periods. Because striatal phasic firing is strongly related to parameters of movement of the correlated body part [direction, apex (position), distance, duration, velocity] (Crutcher and DeLong, 1984; Pederson et al., 1997), comparisons of firing used only straight, vertical head movements that were matched with respect to all these parameters between the two periods.
Pederson et al. (1997) briefly described effects of cocaine on FR of DLS neurons correlated with vertical head movement. However, that study lacked a vehicle control. We now provide this necessary control group of 33 neurons and add 36% more neurons to the high-dose group, plus detailed descriptions of methods not presented in the brief report. Since publication of that study, we have refined post hoc procedures 1) that further restrict variability in all the above movement parameters simultaneously during each movement for which FR is compared between predrug and cocaine periods, and 2) that enable many more of these comparisons for each neuron. Using all the neurons from the earlier and new datasets, we have analyzed substantially more of the voluminous data generated by this paradigm using hierarchical linear modeling (HLM) (Raudenbush and Bryk, 2002). HLM enabled modeling of each neuron's different FR during multiple movements simultaneously with the aggregated FR of all the neurons across all the doses. HLM uses all the discrete data, unlike analysis of variance (ANOVA)/regression, which would require collapsing data within each neuron to avoid type I errors. The unified, comprehensive analysis revealed new dose- and FR-dependent effects of cocaine at both the level of the population of neurons and the individual neuron, enabling a new evaluation, not possible in the earlier study, of current hypotheses regarding DA agonist effects on MSN firing.
Materials and Methods
Subjects.
Forty-eight adult male Long-Evans rats (Charles River Laboratories, Inc., Wilmington, MA) weighing 250 to 350 g underwent surgical implantation, successfully completed all the phases of the study and yielded neural data that met all the criteria. Neural and behavioral data for analysis were contributed by only these 48 animals. To be successful, animals exhibited instrumental head movements and stable single unit recordings throughout predrug, cocaine, and reversal/recovery periods. Animals were randomly assigned to one of four groups, each of which was injected intraperitoneally with a different dose of cocaine (cocaine hydrochloride, supplied by the National Institute on Drug Abuse, Bethesda, MD). Seventeen rats received the control dose (Dose 0), i.e., a saline injection; 8 rats received the 5-mg/kg dose (Dose 5); 10 rats received the 10-mg/kg dose (Dose 10); and 13 rats received the 20-mg/kg dose (Dose 20). Each rat received only one injection of cocaine (or saline in the Dose 0 group). Rats were maintained on a 12:12-h reversed light/dark cycle (on 8:00 PM, off 8:00 AM) so that experiments were conducted during the rats' active phase.
Surgery.
General details of the surgical procedures have been described previously (Carelli and West, 1991). Specific details regarding surgical implantation of the microdrive base or of the microwire array are described below. Animals were allowed 7 to 10 days to recover from surgery before recording began.
Apparatus.
All the experiments were performed in a clear Plexiglas chamber (35 ×17 × 40 cm). A commutator (Crist Instrument Co., Inc., Hagerstown, MD) was placed in the center of the chamber's ceiling. A speaker that delivered an audible tone (750 Hz, 65 dB, 50 ms) to signal the delivery of each water reward (see below) was placed on the ceiling facing downward into the chamber.
A Plexiglas apparatus inserted into the chamber was designed to keep the rat's body straight with respect to the camera and also to prevent the rat's harness from striking the walls of the chamber during the experimental session. The Plexiglas apparatus consisted of 4-cm-high walls on left and right sides of the rat that sloped down from the walls to the floor at a 45° angle. These sloping sidewalls extended from the front of the chamber toward the back wall, and were 20 cm in length, i.e., the entire length of the rat's body. In effect, the Plexiglas apparatus formed a crevice 8-cm wide at the floor that centered the rat facing the camera while performing the experimental task. At the front of the chamber, the Plexiglas apparatus supported a water trough centered 8.5 cm from each side wall, raised 1.5 cm from the floor, and extending 6.5 cm from the front wall.
At the beginning of each experimental session, before the rat was put into the chamber, a false floor made of black Plexiglas was placed over the Plexiglas apparatus such that it covered the water trough. The false floor was removed when the experimental session began. A speaker in the ceiling of the room that housed the experimental chamber delivered white noise during the experimental session to minimize auditory distractions.
The animal was connected to the recording equipment via a harness sheathed in a strong but flexible wire spring leash (Peoples and West, 1996) that prevented the rat from biting the wires inside. The harness' base (headstage) contained field effect transistors, and the top of the harness was connected to the commutator. Extracellular action potentials from the electrodes were carried through the harness and commutator system to a preamplifier (gain = 10), a bandpass filter/amplifier (roll-off less than 1000 Hz = 1.5 dB/octave and greater than 11,000 Hz = 6 dB/octave; gain = 500) and then to a computer that used Datawave Discovery acquisition software (Datawave Systems Corp., Longmont, CO), which digitized (50 kHz) and time-stamped extracellular action potentials for offline analysis.
Two light-emitting diodes (LEDs) were embedded 9 mm apart vertically in the base of the harness. The LEDs were precisely aligned so that they faced directly forward, parallel with the long axis of the rat's body. The bottom LED was flanked on the left and right by “blinders” such that any head movements exhibiting a horizontal deviation of more than 20° away from the camera on either side were not processed and were discarded. This ensured that all the recorded movements occurred while the rat was facing the camera, without horizontal turning of its head (long axes of the rat's body and head were parallel). The top LED was used to monitor parameters of each movement (see below).
During the experimental session, all head movements were tracked by monitoring the two LEDs with a camera (Computar series 3500; CBC Corp., Commack, NY) that faced the animal and was located outside the front Plexiglas wall. The positions of the two LEDs were detected with a spatial resolution of 1 mm (given that the animal stood approximately 25 cm from the camera's lens). The LED signals from the camera were processed by a diode “tracker box” with a 60-Hz sampling rate (16.7-ms temporal resolution; Datawave Systems Corp.). On the vertical scale, a value of 0 corresponded to the floor of the chamber. A value of approximately 90 to 95 mm for the upper LED corresponded to a level position of the animal's head, i.e., neither lowered nor elevated, while the animal was standing on all fours. The digitized LED signals from the diode tracker were sent to the computer, where their position information was integrated with the neural waveform information by the Datawave Discovery program for post hoc analysis. The tracker box enabled the adjustment of the threshold brightness level at which the LEDs were detected by the computer, allowing enough room lighting for simultaneous video recording of the animal.
In all the experimental sessions, the video signal from the camera was also sent to a videocassette recorder (Sony Super Beta SL-HF750 or Sony VHS SLV-789HF; Sony Corp. of America, New York, NY). Datawave Discovery (Datawave Systems Corp.) time-stamped each frame of the video recording at a resolution of 30 frames/s (33 ms between frames) by way of a video frame counter (model VC-436; Thalner Electronics Laboratories, Ann Arbor, MI). Video recordings were used post hoc 1) to assess times when the rat performed the task but neglected to collect water rewards, and 2) to eliminate from analysis infrequent times when the rat was off the operant task or when the long axes of the rat's body and head were not parallel.
Microdrive Recording.
Animals at Doses 5, 10, and 20 mg/kg were surgically prepared with a microdrive base (Crist Instrument Co., Inc.) over the right lateral striatum, 0.2 anteroposterior and 3.5 mediolateral in relation to bregma (level skull). Two connector strips (ITT Cannon, Santa Ana, CA) were implanted perpendicular to the long axis of the animal and were cemented at a standard height such that the top of the strips was positioned 12 mm above the posterior portion of the skull. Thus, after inserting the harness into the connector strips, the position of the LEDs was uniform across subjects.
Before each experimental session, a microdrive housing a tungsten microelectrode (1–10 MΩ; FHC Inc., Bowdoin, ME) was mounted onto the microdrive base without the use of anesthetic. The microdrive enabled the electrode to be lowered into the brain without rotating it (400 μm/manual rotation), thus minimizing tissue damage. The harness was connected to the animal, and an electrode track profile was created (Carelli and West, 1991) to ensure that only striatal neurons were recorded during the session. When the electrode entered a “quiet zone,” which corresponds to white matter of the corpus callosum (Carelli and West, 1991), it was allowed to settle for approximately 3 h. After settling, the electrode was advanced into the striatum. When a neuron was encountered that appeared to show a correlation with vertical head movement, a full sensorimotor examination was conducted (see below). Once it was verified that the neuron was correlated exclusively with vertical head movement, the electrode was allowed to settle for at least 30 min before beginning the experimental session. Microdrive recordings yielded 75 neurons to the present dataset, most of which were in the dataset briefly reported by Pederson et al. (1997).
Microwire Recording.
The rats at Dose 0 were chronically implanted with a 6 × 2 array of quad-Teflon-coated stainless steel wires (50-μm diameter without insulation, 0.2 MΩ; California Fine Wire Company, Grover Beach, CA). These were attached to two connector strips for the harness (Microtech Inc., Boothwyn, PA), which were cemented perpendicular to the long axis of the animal. The strips were implanted at a standard height: the top of the strips was positioned 12 mm above the posterior portion of the skull. Thus, after inserting the harness into the connector strips, the position of the LEDs was uniform across subjects. Further specifications of the array have been described previously (Peoples and West, 1996). Coordinates of the targeted area for microwire tips were 1.5 to −0.4 anteroposterior, 3.2 to 4.2 mediolateral, and 3.5 to 4.5 dorsoventral in relation to bregma, to maximize the likelihood of recording striatal neurons related to head movement. Microwire technology, available only for rats in the Dose 0 group, which was the final dose group of the experiment (yield = 33 neurons), was used because 1) it allowed an increased number of recorded neurons per animal relative to the microdrive preparation, and 2) it virtually eliminated the risk of losing neuronal signals as a result of electrode movement during injections. In contrast, such movement of microelectrodes in microdrive recordings resulted in having to discard data from a large number of animals (see under Results). Parameters of extracellular action potential waveforms of striatal neurons were similar whether recorded by stainless steel microwires or tungsten microelectrodes using the microdrive; therefore, data collected from both types of electrode were pooled.
Preliminary Sensorimotor Examination.
A sensorimotor examination, as described previously (Carelli and West, 1991), was conducted before the experimental session for each neuron recorded to verify that firing related to vertical head movement was not also correlated with movement and/or somatosensory stimulation of any other body part. If the neuron also exhibited at least a 3:1 signal-to-noise ratio, it was recorded during the experimental session.
Procedure.
After rats recovered from surgery, the animals were gradually shifted to a water deprivation schedule that lowered their intake of water until they reached 82 to 84% of ad lib body weight. They were allowed free access to food.
Before the day of the experimental session, rats were conditioned in the Plexiglas recording chamber for 2 to 4 h to emit upward head movements. An upward head movement was defined as a “criterion” movement if it exhibited a length of at least 41 mm and started below a prescribed level (94 mm; head even with body, neither raised nor lowered). A criterion movement caused the immediate delivery of a water drop (3 μl) to the trough via activation of a solenoid valve coincident with an auditory tone (3.5 kHz) delivered by the speaker. All the rats achieved a fixed ratio schedule ranging from FR1 to FR5 criterion head movements during this initial training. Thus, in the next session (the experimental session), they had acquired the instrumental response but had not acquired a habit of head movement, and emitted more than 1000 criterion head movements, as well as several thousand, noncriterion vertical head movements.
The maximum total weight of the implant on the skull, including the mounted microdrive, and the recording harness while it was plugged into the commutator was determined to be approximately 12 g. The normal weight of the rat's head was determined to be 60 g. Thus, the combined weight of the implant, the recording harness while connected to the commutator, and the rat's head was determined to be approximately 72 g. Therefore, during each head movement in the experiment, the rat moved approximately 20% more weight than without the surgical implant.
The experimental session comprised three time periods for rats at Doses 5, 10, and 20. The first (T1) was a predrug period of 15 to 60 min that began with an intraperitoneal injection of saline (0.2 ml). The second period (T2) began with an intraperitoneal injection of cocaine 5, 10, or 20 mg/kg (0.2–0.4 ml), lasting 1 h. The third period (T3) was run to permit an analysis of reversal or recovery of the effects of cocaine injection. T3 continued for an additional 60 to 90 min or until the rat ceased responding because of satiation or there was a loss of neuronal isolation. For rats at Dose 0, the experimental session comprised time periods T1 and T2, which began with an intraperitoneal injection of saline. For all the animals, neural and behavioral data were collected from only this one experimental session.
Histology.
After the experiment, the rat was anesthetized with a lethal dose of pentobarbital sodium (150 mg/kg, i.p.). For the rats implanted with a microdrive base, a 0.010 stainless steel insulated wire was mounted in the microdrive and lowered to the depth in the lateral striatum at which the neuron had been recorded (Carelli and West, 1991). Current (30 μA, 10 s) was passed through the wire to make a lesion. For the rats implanted with a microwire array, anodal current (50 μA, 4 s) was passed through each wire to make a lesion. Details regarding removal of the brain and slicing for histological verification of the placement of recording wires have been described previously (Peoples and West, 1996). Any recordings corresponding to lesion locations outside the striatum were discarded.
Analysis of Head Movement.
Cocaine-induced stereotypy.
Stereotypy was operationalized as the total time that the rat continued emitting operant responses (criterion head movements) in T2 without collecting the water drops that were being delivered. The duration of this behavior was measured using offline videotape analysis and was analyzed using ANOVA.
To assess whether responding exhibited an extinction pattern, each animal's rate of responding throughout the duration of this behavior was assessed by dividing the duration (which differed among animals) into quartiles and calculating the rate of responding per minute during each quartile. Rates from each quartile were analyzed using a mixed model ANOVA in SAS PROC GLIMMIX (SAS version 9.1.2; SAS Institute, Cary, NC) in which a gamma distribution with a log link function was specified for the outcome variable. The final solution for the mixed ANOVA model was estimated using maximum pseudo-likelihood marginal expansion. The degrees of freedom in the model were computed using the containment method. Standard errors were computed using the first-order residual empirical estimator, also known as the sandwich estimator. All the other default settings in PROC GLIMMIX were maintained.
Behavioral parameters.
Behavioral parameters were calculated based on the digitized record of movement of the top LED on the harness and were quantified using an x-y Cartesian coordinate system superimposed on the camera's view of the chamber. For behavioral data analysis, a vertical head movement was defined as any vertical head motion of at least 4 mm. The beginning and end of a movement were defined by either a change in direction, e.g., the end of a downward movement was the beginning of an upward movement, or by a pause of at least 33 ms, e.g., if an upward head movement paused for at least 33 ms and then resumed, the beginning of the pause defined the end of one upward head movement and the end of the pause defined the beginning of another upward head movement. Note that the definition of a vertical head movement for behavioral data analysis was more liberal than for neural data analysis (see below). This ensured that the effects of cocaine on behavior could be accurately observed over a wide range of movements while at the same time neural analysis was focused within movement parameters to which each neuron was sensitive, in particular, direction.
During long vertical head movements, as a result of the pivoting of the rat's head on its neck, the LEDs on the harness arced away from the camera in a third Cartesian “z” dimension (depth), which the camera could not directly observe. To prevent distortion of the observed vertical y-component of each movement, y-coordinates were adjusted post hoc by the addition of constant values that were calculated based on trigonometric ratios. These constants progressively increased for higher y-coordinates and allowed for a more accurate representation of position along the entire arc of the movement.
Each vertical head movement was quantified with respect to several different measures operationalized as follows. Apex was the highest point and valley was the lowest point, in millimeters, as defined in the y-dimension. Distance between the valley and apex, in millimeters, was calculated according to the Pythagorean theorem, as the hypotenuse of the triangle with the horizontal component (x-dimension) and the vertical component (y-dimension) of the head movement's trajectory. Duration was the time, in milliseconds, from the beginning to the end of vertical movement. Average velocity was calculated as distance/duration.
Neural Analysis.
Waveform discrimination and perievent time histogram analysis.
As described previously (Carelli and West, 1991; Peoples and West, 1996), neural waveforms were processed and isolated through Datawave Analysis software (Datawave Systems Corp.). To verify that a single neuron was isolated, the interspike interval was required to show a 2-ms period after each discharge that was devoid of discharges, consistent with the natural refractory period of a single neuron.
Of 86 neurons undergoing interspike interval analysis, 79 (92%) met this criterion. The remaining 22 neurons' recordings were of similar quality but were not tested. Each neuron's set of waveforms was also required to show stability in various waveform parameters, such as amplitude and peak. If waveform parameters of a neuron changed over the course of the experimental session, analysis of the neuron's data was terminated at the time point corresponding to the beginning of the change, or the neuron's data were completely removed from the data pool.
Effect of cocaine on neural waveforms.
A concern was that the high dose (20 mg/kg) could potentially alter the shape of a neuron's waveform if some sodium channels were blocked by cocaine, i.e., a local anesthetic effect. Reduction of sodium current could lead to a decrease in the amplitude of the waveform and inconsistent detection, resulting in incorrect computation of the FR for that neuron during T2. This concern was addressed by analyzing whether the waveform shape was altered by cocaine. The waveform parameters of amplitude and peak time were extracted using the Datawave Parameter Extraction module (Datawave Systems Corp.) for the six neurons that showed the greatest suppression postinjection in the Dose 20 group. The median was then calculated for all the values of amplitude and peak time within a neuron for T1 and T2, and the medians were then analyzed in a repeated-measures ANOVA.
Strategy.
Striatal neurons exhibit unconditioned correlations with sensorimotor activity of specific body parts (Crutcher and DeLong, 1984; Carelli and West, 1991). Studying them requires recognition of their sensitivity to sensorimotor variables at several levels. Each neuron fires selectively 1) at the first level, with respect to one specific body part; 2) at the second level, with respect to the direction of activity of that body part; 3) at the third level, with respect to parameters of sensation or movement in that body part's preferred direction, such as distance, velocity, and so on. Our strategy was to study firing during the same movement both before and after injection (see Trulson and Jacobs, 1979). “Behavioral equivalence” has been used to eliminate confounds from interpretations of cocaine-induced changes in other neural measures (Porrino, 1993). Without proper controls, subtle differences in movement between the predrug and drug periods could result in changes in FR (e.g., related to feedback from movement altered by systemic drug effects) that may be mistakenly interpreted as pharmacological effects on recorded neurons. To remove these concerns, we have studied stimulant effects on neurons related to head movement during head movement before and after injection (West et al., 1997). Only neurons related specifically to vertical head movement were selected for the present study, and all the assessments of FR differences between predrug and drug periods involved precisely matched, vertical head movements based on approximately 20 parameters of each movement, leaving any differences in FR to be interpreted as drug-related. Because this matching was performed post hoc, the design allowed the animal's movements, as well as cocaine's motor effects, to be unrestricted. Each rat generated 10,000 to 20,000 vertical head movements for post hoc analyses.
Matched pairs.
Neurons related to vertical head movement are directional and can also be correlated with any combination of apex (position), distance, duration, or velocity of head movement (Pederson et al., 1997). Therefore, analyses of each neuron's FR took all the potentially correlated parameters into account via the following procedure.
For every neuron, the correlation of firing with vertical head movement was determined via the experimenter's sensorimotor examination (above) before the session and through the post hoc construction of perievent time histograms (PETHs). Two PETHs were constructed around two different nodes: beginning of upward head movement and beginning of downward head movement, with a time base of 1 s before and after the node. The direction with which firing was correlated was determined from the PETHs and the sensorimotor examination. Only neurons that both satisfied the criteria used in the sensorimotor examination and exhibited a correlation with vertical head movement via one of the two PETHs were put into the data pool for neural analysis.
Post hoc, refinement of the dataset for neural analysis began by filtering out all the movements that were slanted, curved, crooked, and so on, so that the refined dataset contained only head movements that were uniformly straight and vertical. A straight and vertical head movement was defined as having slant ratios no greater than 0.25 (ratio of horizontal to vertical displacement) and curvature ratios no greater than 0.15 on either side of the movement. In addition, all the movements for the neural analysis were at least 4 mm in distance and at least 30 ms in duration. Requiring a minimum distance and duration eliminated from neural analysis extremely short head movements or jitter between camera pixels. Note that the definition of a vertical head movement for neural data analysis was more restricted than that for behavioral data analysis.
Next, the behavioral parameters of apex, distance, and duration were blocked into 10 levels spanning their full range. The resolution of the 10 bins for apex and distance was 6 mm, and the resolution for the 10 bins of duration was 33 ms. The minimum value of apex was 172 mm, with the final, i.e., 10th, category being a catch-all category for movements with an apex ≥226 mm. The minimum value of distance was 4 mm, with the final category being a catch-all category for movements with a distance ≥58 mm. The minimum value of duration was 16 ms, with the final category being a catch-all category for movements with a duration ≥301. Velocity was implicitly blocked because it was calculated as distance/duration. The blocked parameters were then used to create a three-dimensional matrix (3D; 10 × 10 × 10 cells), one for T1 and another for T2, with each blocked parameter represented as an axis. Using the refined dataset, every movement in T1 and T2 was classified according to its distance, duration, and apex for assignment to the single, appropriate cell in the T1 or T2 3D matrix. Each cell in the T1 3D matrix and its counterpart in the T2 3D matrix were defined as a “matched pair” (see Table 1). In any matched pair, variance of every movement parameter was narrowly restricted simultaneously with all the other parameters, such that comparing T1FR with T2FR involved virtually identical movements from the two time periods.
Table 1.
An example of the creation of 16 matched pairs of one representative striatal neuron's firing during three experimental periods (predrug, cocaine, recovery/reversal) in the medium cocaine dose (10 mg/kg) group using statistical blocking
Left half of table represents a 2D slice of the 3D head movement-blocking matrix (see text) comprising 10 levels of distance and 10 levels of duration at one level of apex (220 mm < apex ≤ 225 mm) for one representative animal at each of the three periods. Each categorical level was labeled by the maximum of the range of parameter values assigned to it, e.g., a distance level of 21 had a range of (15 mm < distance ≤ 21 mm). Right half shows the firing rates associated with all the blocking matrix cells in the left half that contained enough movements (n ≥ 5) to create a matched pair. The bolded and underlined entries constitute an example of a particular set of cells (distance = 21 mm, duration = 100 ms, apex = 225 mm) in the 3D blocking matrix that yield a matched pair for the T1–T2 analysis of the effects of cocaine on firing rate (T1FR = 2.4 discharges/s, T2FR = 3.1 discharges/s) and another matched pair for the T1–T3 analysis of recovery/reversal (T1FR = 2.4 discharges/s, T3FR = 1.1 discharges/s).
| Time Period | Head Movement Duration | Number of Head Movements |
Head Movement Duration | Mean Firing Rate (discharges/s) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head Movement Distance (mm) |
Head Movement Distance (mm) |
|||||||||||||||||||||
| 9 | 15 | 21 | 27 | 33 | 39 | 45 | 51 | 57 | 58+ | 9 | 15 | 21 | 27 | 33 | 39 | 45 | 51 | 57 | 58+ | |||
| ms | ms | |||||||||||||||||||||
| T1 | 33 | 8 | 33 | 0.0 | ||||||||||||||||||
| Predrug | 67 | 25 | 17 | 6 | 6 | 67 | 0.0 | 3.3 | 7.7 | 0.0 | ||||||||||||
| 100 | 10 | 20 | 10 | 100 | 2.4 | 0.0 | 0.0 | |||||||||||||||
| 133 | 9 | 20 | 12 | 13 | 16 | 133 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||||
| 167 | 57 | 167 | 0.3 | |||||||||||||||||||
| 200 | 32 | 200 | 0.3 | |||||||||||||||||||
| 233 | 36 | 233 | 0.9 | |||||||||||||||||||
| 267 | 267 | |||||||||||||||||||||
| 300 | 300 | |||||||||||||||||||||
| 301+ | 301+ | |||||||||||||||||||||
| T2 | 33 | 5 | 33 | 0.0 | ||||||||||||||||||
| Cocaine | 67 | 27 | 20 | 16 | 18 | 67 | 3.0 | 6.2 | 0.0 | 1.1 | ||||||||||||
| 100 | 12 | 14 | 5 | 100 | 3.1 | 11.9 | 0.0 | |||||||||||||||
| 133 | 5 | 8 | 8 | 11 | 8 | 133 | 3.0 | 1.0 | 3.3 | 4.6 | 1.0 | |||||||||||
| 167 | 10 | 167 | 1.3 | |||||||||||||||||||
| 200 | 11 | 200 | 3.3 | |||||||||||||||||||
| 233 | 5 | 233 | 0.0 | |||||||||||||||||||
| 267 | 267 | |||||||||||||||||||||
| 300 | 300 | |||||||||||||||||||||
| 301+ | 301+ | |||||||||||||||||||||
| T3 | 33 | 13 | 33 | 0.0 | ||||||||||||||||||
| Recovery/Reversal | 67 | 54 | 38 | 13 | 16 | 67 | 0.8 | 1.4 | 0.0 | 2.6 | ||||||||||||
| 100 | 31 | 33 | 12 | 100 | 1.1 | 0.3 | 0.0 | |||||||||||||||
| 133 | 13 | 12 | 15 | 17 | 56 | 133 | 0.0 | 0 | 0.0 | 0.5 | 0.3 | |||||||||||
| 167 | 109 | 167 | 0.1 | |||||||||||||||||||
| 200 | 70 | 200 | 0.1 | |||||||||||||||||||
| 233 | 45 | 233 | 0.2 | |||||||||||||||||||
| 267 | 267 | |||||||||||||||||||||
| 300 | 300 | |||||||||||||||||||||
| 301+ | 301+ | |||||||||||||||||||||
FR was calculated for each movement and then averaged for all the movements within each cell in each rat's T1 and T2 3D matrices. We used FR analysis rather than spike train analysis because few movements contained more than one discharge. FRs greater than 1.0 were obtained because movement duration (the denominator used in calculating FR) was always less than 1 s. FR from each matched pair was used for subsequent analysis only if both cells in a matched pair each contained at least five movements. A partial table of matched pairs for one neuron is shown in Table 1 for the 10 levels of distance, 10 levels of duration, and one of the 10 levels of apex at three time periods. FRs in these matched pairs, i.e., during similar sets of movements, were the only FRs used in the analysis of T1 versus T2 differences in FR, i.e., were the only data used in assessing cocaine's effects on FR.
The above procedure was repeated for the T1–T3 data to create matched pairs for the assessment of reversal/recovery from drug effects on firing (see below). T1–T3 matched pairs were created for cells that contained at least five movements in each respective period and whose T1–T2 counterpart matched pairs were included in the T1–T2 dataset.
Four important advances have been achieved since the earlier publication by Pederson et al. (1997), mostly stemming from our more recent development of generating the 3D matrix for each neuron. First, using the 3D matrix, extensive analyses of the relationship of FR to direction, distance, duration, apex (position), and velocity have not only substantiated their importance but also ruled out other parameters (e.g., time between movements) among the 20 parameters of head movement measured by the 16.7-ms resolution of the present diode-tracking technique. Still other parameters are now used to filter out certain movements to restrict and refine the dataset for neural analysis (e.g., slant, curvature, criss-cross; see above). Second, using the 3D matrices, the number of independent observations of each neuron's FR was substantially increased over the one to four observations per neuron available for the earlier study. Third, detailed analyses (data not shown) revealed that the criterion of using five or more movements for each matched pair yielded similar accuracy in assessing FR of all 108 neurons as was observed using a minimum of 10, 15, or 20 movements (a minimum of 20 was used by Pederson et al., 1997). Using the criterion of five or more movements enabled many more of the cells of the 3D matrix to be used in neural analysis, i.e., yielded substantially more data points (matched pairs) for each neuron. The increase in the number of independent observations by these two advances not only added to the power of statistical analyses but also enabled detailed analyses of cocaine's effects on individual neurons that were not possible in the earlier study. Fourth, all the observations of FR for each neuron across all neurons and all doses were able to be simultaneously analyzed using HLM (see below), yielding new insights that could not have been recognized in our earlier study.
Analysis of Drug-Induced Changes in Firing.
HLM.
The matched-pairs electrophysiological data were clearly hierarchical in that the FRs of the matched pairs were nested within neurons, which created a two-level hierarchy. Statistical analysis of hierarchical data needs to take into account the nesting associated with any hierarchy present within the data to avoid a severely inflated type I error rate (Raudenbush and Bryk, 2002; Stevens, 2002). HLM (Raudenbush and Bryk, 2002) was used to analyze the FRs of the matched pairs because it is a well developed statistical technique that can take into account the nesting of data (Raudenbush and Bryk, 2002). The software program HLM6 (Scientific Software International, Inc., Lincolnwood, IL) was used to carry out the HLM analysis. The solution was estimated using restricted maximum likelihood and specifying the matched-pair FRs within each neuron as “measures within person” (Raudenbush and Bryk, 2002).
Before the HLM analysis was carried out, the intraclass correlation was computed for the dependent variable of interest (T2FR) using the procedure outlined on page 71 in Raudenbush and Bryk (2002). The intraclass correlation is a measure of the proportion of variance explained only by the nesting variable, which in this case is neurons (Raudenbush and Bryk, 2002; Stevens, 2002). A larger intraclass correlation indicates a greater degree of violation of the assumption of independence as a result of nesting among the observations of the dependent variable, and therefore a concomitant increase in the type I error rate if the nesting variable is not appropriately taken into account in any statistical analysis (Stevens, 2002).
For the HLM analysis, neurons were defined as the nests. Matched pairs nested within individual neurons formed the first level (level 1), and neurons formed the second level (level 2). The level 1 model will be referred to as the “within-neuron” model, and the level 2 model will be referred to as the “between-neuron” model. The within-neuron model was a linear regression of T2FR on the centered T1FR of the matched pairs for each individual neuron. For the within-neuron model, group mean centering was performed, which involved subtracting the average T1FR of a given neuron from the T1FR values of all that neuron's matched pairs. Because of the group mean centering transformation, the within-neuron intercept was mathematically equivalent to a neuron's average T2FR (see Raudenbush and Bryk, 2002 for more detail about centering). The final within-neuron model was
where T2FRij is the T2FR of the ith matched pair for the jth neuron, β0j is the centered intercept for the jth neuron, β1j is the slope of the regression of T2FR on centered T1FR of the i-matched pairs for the jth neuron, and eij is the residual error term for the jth neuron.
The between-neuron model used the parameters of the within-neuron model (β0j, β1j) as dependent variables. The between-neuron model contained the predictor variables of dose, dose2 and average T1FR of each neuron, and the interactions of dose · average T1FR of each neuron and dose2 · average T1FR of each neuron. Dose and average T1FR were grand mean-centered, which involved subtracting the grand mean of a variable from all the values of that variable (Raudenbush and Bryk, 2002). Centering of the between-neuron variables reduced the multicollinearity that can arise from the creation of interaction terms in regression models (Raudenbush and Bryk, 2002). The final between-neuron model was
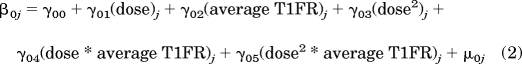 |
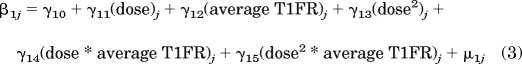 |
where β0j is the intercept for the jth neuron and β1j is the slope for the jth neuron from the within-neuron model. Because of the centering procedures used in the within- and between-neuron models, the between-neuron intercept parameters of γ00 and γ10 correspond to the average within-neuron intercept and average within-neuron slope, respectively. All the other γ parameters are the slope coefficients associated with the between-neuron independent variables. The μ parameters are residuals that model the unexplained portion of the between-neuron variance. The proportion of variance of both the within-neuron intercept and within-neuron slope that is explained by the between-neuron predictors was computed using a procedure outlined on page 85 in Raudenbush and Bryk (2002). Because the distribution of T2FR was non-normal, robust S.E. were used because they are less sensitive to departures from normality than normal S.E. (Raudenbush and Bryk, 2002). A pilot analysis also determined that there was no statistical difference between the within-neuron mean and median T2FR, which indicated that the mean was essentially an accurate representation of the central tendency of each neuron's T2FR despite the non-normal distribution of T2FR.
Within-neuron intercept (average T2FR).
The results of the within-neuron intercept (β0j) portion of the HLM were complemented by additional graphical and statistical techniques. First, the proportion of variability of the within-neuron intercept (β0j) explained exclusively by the average T1FR at the between-neuron level, irrespective of dose, was calculated using the procedure outlined on pages 74 through 75 in Raudenbush and Bryk (2002). Second, the empirical Bayesian (EB) estimates derived from the HLM (see Raudenbush and Bryk, 2002, pages 46 and 268–269) of the within-neuron intercepts, i.e., the average T2FR, were plotted against the corresponding values of raw average T1FR for each dose. Third, the EB estimates of average T2FR were linearly regressed, using ordinary least squares (OLS), on the raw average T1FR for each dose to determine the test-retest stability of average FR per each dose. Logarithmic scaling was used for the plotting and the raw data were log-10 transformed for the associated OLS regression analyses of T1FR and T2FR to better illustrate slower-firing neurons.
Fourth, for each neuron, the percentages were computed of 1) matched pairs that exhibited a FR of zero in T1 and a nonzero value in T2 (elevation from 0), and 2) matched pairs that exhibited a nonzero FR in T1 and a value of zero in T2 (suppression to 0). Analysis of covariance (ANCOVA) was used to separately analyze both these percentages in SAS PROC MIXED. All the percentages were transformed using the formula.
Dose and average T1FR of each neuron were specified as the main effects, and the interaction between the two independent variables was also specified. Robust S.E. was specified using the “empirical” option in PROC MIXED because the distribution of the dependent variable was non-normal. All the other default settings in PROC MIXED were maintained. Planned simple-effects comparisons were specified to be run using PROC MIXED only if the two-way interaction in the ANCOVA was statistically significant at the 0.05 level. The planned simple effects consisted of systematically testing the difference between the Dose 0 control group and the other dose groups at the low and high ends of the interquartile range, i.e., the 25 and 75 percentiles, of the average T1FR for all the neurons (0.58 and 5.89 discharges/s). Each set of tests between the control group and the three other dose groups was considered a family of statistical tests, for a total of two families. Overall type I error was controlled by using the Dunnett's correction within each family of comparisons and declaring significance at the 0.05 level with the Dunnett's adjusted p value. All the reported p values are Dunnett's-adjusted.
Within-neuron slope.
The results of the within-neuron slope (β1j) portion of the HLM were complemented by several additional graphical and statistical techniques. The proportion of variance of the within-neuron T2FR of matched pairs, explained exclusively by the T1FR of matched pairs at the within-neuron level, was calculated using the procedure outlined on pages 78 through 80 in Raudenbush and Bryk (2002). This result can be compared with the proportion of variability of the within-neuron intercept explained exclusively by average T1FR at the between-neuron level (see above) to determine whether T1FR has a stronger relationship with T2FR at the within-neuron level or at the between-neuron level (Raudenbush and Bryk, 2002).
HLM is a powerful and flexible technique, but it suffers from a limitation that is common to all the regression techniques in that different patterns of data can yield similar regression slopes. As a result, HLM could potentially miss certain differences in the pattern of change of FR from T1–T2, as measured by the within-neuron slope, across different levels of dose or average T1FR. This limitation was addressed by a graphical analysis using visual inspection of scatter plots of matched pairs in which T2FR was regressed on T1FR for each individual neuron.
Two analytical strategies were developed to efficiently present the salient patterns of FR in T1, T2, and in the change from T1 to T2 that were observed throughout the scatter plots, especially across different levels of dose and average T1FR. First, an R2 was calculated for each neuron to determine the amount of variance of T2FR explained by T1FR for each neuron. The R2 values also measured the stability of the matched pair FR from T1 to T2 for each neuron. ANOVA was used to test whether the R2 values for each neuron significantly differed across dose. A second strategy is described in the next section.
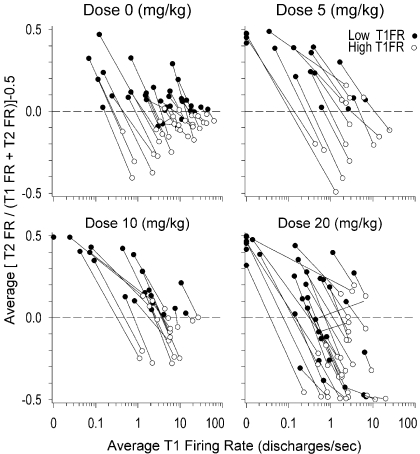
Within-neuron magnitude of change in FR from T1 to T2.
An analysis was developed that specifically focused on quantifying the within-neuron magnitude of change in FR from T1 to T2. For this analysis, we calculated a standardized measure of change for the T1FR and T2FR of each matched pair. The formula for the standardized measure of change was {[T2FR/(T1FR + T2FR)] − 0.5}, which gave a value of 0 if no change occurred between T1 and T2, a value greater than 0 (to a maximum of 0.5) if there was an increase in firing from T1 to T2, and a value less than 0 (to a minimum of −0.5) if there was a decrease in FR from T1 to T2. Because this transformation was inappropriate for matched pairs with a FR of 0 discharges/s, a constant of 0.01, the smallest decimal increment in FR observed across neurons, was added to the T1FR and T2FR of all the matched pairs to include all the matched pairs into this analysis. To compare the standardized measure of change in firing of matched pairs that have low versus high T1FR relative to all the matched pairs within the same neuron, the T1FR values of matched pairs were split into two groups using as a cut point the average T1FR for each neuron. Matched pairs with a T1FR that was less than or equal to the average T1FR for their neuron were classified as low FR, and matched pairs with a T1FR that was greater than the average T1FR were classified as high FR. Seven neurons exhibited matched pairs with all the T1FR values equal to 0, and these neurons were all classified as exhibiting only low T1FR.
The values of the standardized measure of change for the matched pairs were analyzed using a repeated-measures nested ANCOVA in SAS PROC MIXED, in which dose was specified as a categorical between-subjects independent variable, the matched pair T1FR level (low and high) was specified as a categorical repeated-measures independent variable, the average T1FR for each neuron was specified as a covariate, and all the possible interactions among the three independent variables were specified. The nesting variable for the ANCOVA was the different neurons to which each matched pair belonged. Robust S.E. were specified using the empirical option in PROC MIXED because the distribution of the dependent variable was non-normal. All the other default settings in PROC MIXED were maintained.
Planned simple-effects comparisons were specified to be run using PROC MIXED only if the three-way interaction in the ANCOVA was statistically significant at the 0.05 level. The planned simple effects consisted of systematically testing the difference between the Dose 0 control group and the other dose groups for each possible combination of the low and high levels of the T1FR of matched pairs and three specified levels of average T1FR (0.1, 1, and 10 discharges/s). Each set of tests between the control group and the three other dose groups was considered a family of statistical tests, for a total of six families. Overall type I error was controlled by using the Dunnett's correction within each family of comparisons and declaring significance at the 0.05 level with the Dunnett's adjusted p value. All the reported p values are Dunnett's-adjusted. Additional control of type I error was provided by the ability of PROC MIXED to run the tests of simple effects incorporating the nesting variable of neuron.
Analysis of variability in drug-induced changes in FR.
Previous reports from this laboratory (Pederson et al., 1997; Prokopenko et al., 2004; Tang et al., 2008) have revealed variability between neurons in drug-induced FR changes. Therefore, an analysis of the variability of the standardized measure of change in FR was carried out. Variability across different groups can be assessed by first computing the absolute value of the deviation scores of the observations in a group around a sample median using the formula was = |Yas − Mda|, where was is the deviation score of subject s in group a, Yas is the observation of subject s in group a, and Mda is the median of group a (Abdi, 2007). The values of was can then be analyzed using ANOVA (Abdi, 2007). Because one of the three independent variables, average T1FR, was a covariate, the median could not be straightforwardly computed for the variability analysis. Because the median is typically associated with nonparametric analyses, a nonparametric spline regression curve was fitted to the matched pairs data, and the predicted values based on the spline regression curve were used in place of the median for the computation of was. The spline regression curve was fitted to the entire dataset using SAS PROC LOESS with the three independent variables specified as main effects. The improved Akaike information criterion was used to determine the final form of the spline regression curve. All the other default settings were maintained. The ANCOVA and planned simple effects described above for the standardized measure of change were then rerun on the absolute values of the residuals from the spline regression.
Analysis of recovery from drug effects in T3.
For the animals in the three cocaine groups, neural and behavioral data were recorded for 1 to 2 h past the 1-h cocaine period to assess reversal of, or recovery from, drug effects. T3 data were not collected for Dose 0 because there was no drug effect from which to reverse. Neural data used for analyses of recovery were available for only a subset of the neurons recorded in T1 and T2 (as a result of loss of neural isolation over time in some cases), which led to a reduced dataset for neural recovery analyses. Behavioral data for analysis of recovery were available for all the animals. For all the neural and behavioral analyses described above, post hoc comparisons that differed between T1 and T2, i.e., exhibited an effect of cocaine, were repeated between T1 and T3. In cases where the analysis of recovery and reversal required the use of average T1FR, the average T1FR values based on the original T1–T2 dataset were retained to maintain consistency of neuronal classification between the initial and subsequent recovery and reversal analyses.
Recovery was assessed by rerunning the HLM and replacing T2FRij in equation 1 with the FR of matched pairs in T3 (T3FRij). The EB estimates of average T3FR were linearly regressed on the raw average T1FR for each dose of cocaine using logarithmic scaling. Using the reduced dataset, the HLM of T2FR regressed on T1FR was rerun for comparison purposes. The means and S.D. of the EB estimated slope values from the HLMs of T2FR regressed on T1FR and T3FR regressed on T1FR were computed.
Reversal of the matched pairs for each neuron that exhibited 0 FR in T1 and nonzero FR in T2 (elevation from 0) or that exhibited nonzero FR in T1 and 0 FR in T2 (suppression to 0) was assessed as follows. Matched pairs for all the neurons from both groups (elevation from 0 or suppression to 0) were segregated into two separate sets. Each set was further subdivided into two subgroups: slower- and faster-firing neurons in T1. The cut point for this division was the median of the average T1FR values for all the neurons, which was 1.48. The slower FR subgroup corresponded to the 0.58 discharges/s level, and the higher FR subgroup corresponded to the 5.89 discharges/s level in the post hoc tests for the zero FR MPs described above. It is important to note that the elevation from 0 group had all the T1 matched pairs equal to 0 FR, and the suppression to 0 group had all the T2 matched pairs equal to 0 FR.
The tests of reversal were performed only on the FR from doses and subgroups that exhibited significant post hoc differences from Dose 0 in the percentage of matched pairs that were elevated from 0 FR or were suppressed to 0 FR. Reversal for neurons in the elevation from 0 FR group was assessed by running a nested t test using PROC MIXED on the means of T2FR and T3FR to determine whether T3FR was less than T2FR. Least-square (LS) means and S.E. as computed by PROC MIXED for T2FR and T3FR were reported because the LS means take into account the nested structure of the data. Reversal for the suppression to 0 FR group was assessed by running a one sample nested t test comparing the mean T3FR with a value of zero, which was the T2FR of all the matched pairs in the suppression to 0 FR group.
Results
Of 162 rats implanted, data from 114 rats were not used because 1) neural activity correlated specifically with vertical head movement could not be identified (n = 63); 2) neural activity correlated with head movement was not stable long enough to allow adequate assessment of drug-induced changes (n = 4); 3) behavior on the operant task was compromised, e.g., disrupted after the administration of cocaine (n = 14); or 4) the preparation was compromised because of various technical difficulties concerning the animal's headstage (n = 33). Thus, 48 rats (30%) contributed both neural and behavioral data that met all criteria for analysis.
Behavioral Analysis.
Stereotypy.
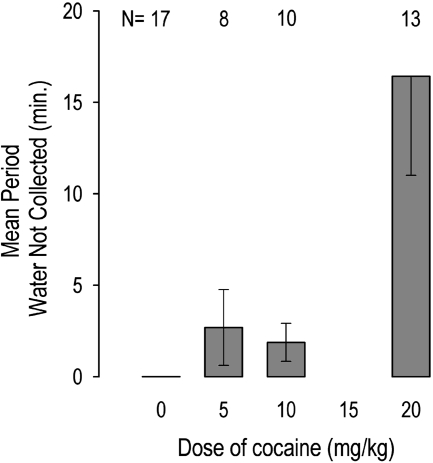
The water reward was collected immediately after each water drop delivery except in the high-dose group during T2. Pederson et al. (1997) reported that rats in the high-dose group failed to collect significant numbers of water reinforcers. In the present study, we added the zero-dose group and analyzed the duration of this effect. Rats at Dose 20 remained “on task,” responding without drinking the earned water drops for up to 1 h, such that water overflowed the trough onto the floor. These animals made no attempt to drink the water but instead engaged in repetitive head bobbing. A between-subjects ANOVA on the time spent in T2 responding without collecting the water reward showed significant differences across the four doses, F(3,44) = 6.74, p < 0.01. Post hoc Tukey honestly significant difference homogeneous group tests showed that Doses 0, 5, and 10 did not differ significantly among themselves but were all significantly different from Dose 20 (p < 0.05) (Fig. 1).
Fig. 1.
Cocaine-induced stereotypy as measured by the number of minutes in cocaine period during which water rewards were earned by emitting criterion head movements but were not collected. Rats at Dose 0 exhibited no stereotypy, i.e., collected all the water rewards. Rats at Dose 20 exhibited highest level of stereotypy.
At Dose 20, responding of the five animals exhibiting the longest durations of this effect (at least 12 min) was analyzed to determine whether it exhibited an extinction pattern, as would be expected if it were maintained by conditioned reinforcement, i.e., the sound of water delivery. Response rates showed no decrease throughout the time of neglecting water delivery, F(3,12) = 0.58, p > 0.05. The quartile means (with S.D. in parentheses) in sequential order were 10.8 (14.6), 9.4 (19.1), 10.1 (16.4), and 11.4 (14.3) responses/min. Thus, responding showed no tendency to extinguish and likely reflects the incorporation of a learned behavior into the stereotypy induced by a stimulant (Ellinwood and Kilbey, 1975). In T3, all the animals resumed collecting water after each delivery (i.e., time spent on task without collecting water in T3 = 0 in all the animals), indicating complete recovery to T1 levels.
Distance.
We previously showed that Doses 5 and 10 (combined) caused an increase in the frequency of long-distance (>40 mm) movements from T1 to T2, which differed from the reduction in long-distance movements caused by Dose 20 (Pederson et al., 1997). As a control for possible changes that might occur spontaneously over time, the present zero-dose group exhibited no change in the frequency of long-distance movements from T1 to T2, t(16) = 0.97; p = 0.76.
Neural Analysis.
No effect of cocaine on neural waveforms.
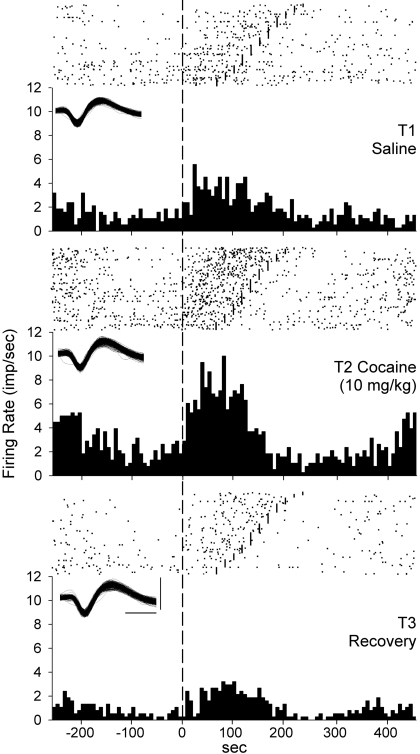
Eight neurons at the high dose whose FRs were most suppressed in T2 were studied to determine whether their waveforms were altered by local anesthetic properties of cocaine, whereby blocking sodium currents could have altered extracellular action potential waveforms, resulting in their less-frequent discrimination. A repeated-measures ANOVA revealed no differences between T1 and T2 in the median values of amplitude (valley-to-peak spike height) for these neurons, F(1,7) = 1.41, p > 0.10. A repeated-measures ANOVA also revealed no differences between T1 and T2 in the median values of peak time for these eight neurons, F(1,7) = 1.00, p > 0.10. Detailed graphic analysis of these eight recordings showed that during T2, waveforms continued to occur infrequently but at the same amplitude as in T1, with no emergence of smaller waveforms. These results indicate that neural waveforms were not altered by the high dose of cocaine. At the medium dose, consistency of waveforms is exemplified in Fig. 6.
Fig. 6.
Changes in FR (impulses/s) across the three periods of a representative single neuron correlated with vertical head movement during head movements in the operant task. Raster plot and corresponding PETH from each period. Each dot in raster represents one discharge. Vertical dashed reference line running from top to bottom: beginning of each upward head movement. Firing is displayed forward and backward in time from movement onset. Short vertical lines in rasters: end of upward head movement on those trials. Discharges at left and right are related to other upward head movements. Trials are sorted from longest to shortest duration vertical head movements. Top, discharges recorded during 588 head movements in T1. Middle, discharges during contiguous block of 588 head movements starting at 30 min after cocaine injection in T2. Bottom, discharges during the last 588 head movements in T3. Insets, overlaid waveforms associated with the discharges graphed in each period. Calibrations: 0.2 mV and 0.2 ms.
Matched pairs.
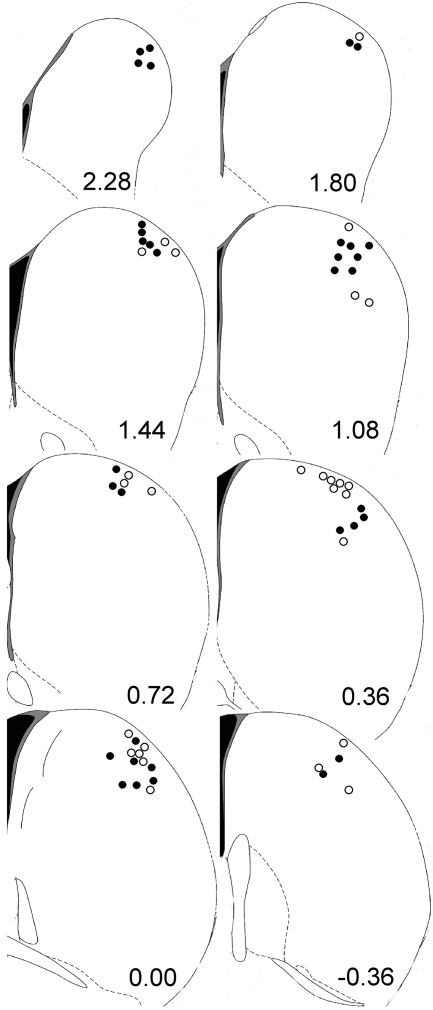
Recordings were obtained from 108 neurons in 48 rats, which yielded 4049 matched pairs: 983 matched pairs from 33 neurons at Dose 0, 1106 matched pairs from 17 neurons at Dose 5, 563 matched pairs from 20 neurons at Dose 10, and 1397 matched pairs from 38 neurons at Dose 20. The median numbers of matched pairs (with the minimum and maximum values in parentheses) obtained per neuron for Doses 0 through 20 were 30 (10, 71), 59 (3, 126), 19.5 (3, 95), and 32.50 (1, 124), respectively. The wire tips that recorded all the neurons reported here were histologically verified to be located in the dorsolateral striatum (Fig. 2). Most neurons exhibited moderate to strong correlations with at least one of the behavioral parameters of apex, distance, and duration, as shown previously (Pederson et al., 1997), illustrating the importance of blocking on these parameters in creating matched pairs to determine the drug effect on firing.
Fig. 2.
Locations of all 108 neurons, which were histologically verified to be in dorsal-lateral striatum. Coronal sections of striatum show anterior-posterior distance of each section from bregma (Paxinos and Watson, 2005). Black dots represent one single neuron recorded; open circles represent the location of two or more single neurons recorded.
All the movement-related firing in the present study is interpreted as reflecting the unconditional “tuning” of these neurons to vertical head movement, and not effects of conditioning. First, training-related changes in firing of these neurons do not occur within one session, as used in the present study, but require several training sessions (Carelli et al., 1997; Tang et al., 2007). Second, only ∼5% of all the head movements for which firing was analyzed activated the tone/solenoid and earned water. Third, the tone/solenoid sounded at or near the end of an upward criterion movement and could not influence firing until near the end of those movements. Fourth, the present type IIB neurons related to head movement exhibit no response to the tone signaling water delivery in this task (see also Carelli et al., 1997; Ma et al., 2009).
Intraclass correlation.
The overall intraclass correlation for T2FR was 0.71, which indicates that there was a large degree of dependence among the observations of T2FR caused by the nesting variable of neuron (Stevens, 2002). The large intraclass correlation also indicates that ignoring the hierarchy present in the data for any statistical analysis would lead to an excessively large increase in type I error rate; therefore, HLM was used (Raudenbush and Bryk, 2002; Stevens, 2002). HLM enabled extraction of multiple electrophysiological data points from each neuron and modeling of individual neurons' FRs simultaneously with the aggregated FRs of all the neurons across all the doses, including a saline control for changes that may occur naturally over the several-hour time course of the experiment.
Dose-and rate-dependent effect of cocaine on average T2FRs.
Tables 2 and 3 present results of modeling the changes in average FR of individual neurons by fitting an HLM on their T1–T2 matched pairs. For the within-neuron level intercept (β0j), i.e., average T2FR, portion of the model, the HLM revealed a significant interaction of dose and average T1FR, γ04 = −0.0322, t(102) = −3.00, p < 0.01, and a significant interaction of dose2 and average T1FR, γ05 = −0.0045, t(102) = −2.23, p < 0.05, on the average T2FR of individual neurons. These results indicate that the relationship between the average T1FR and average T2FR of individual neurons was significantly different across doses. The proportion of variance of the within-neuron intercept that was explained by all the between-neuron predictors was 0.93. The proportion of variance of the within-neuron intercept explained by the average T1FR alone was 0.69, which indicates that, across neurons, average T1FR was a powerful predictor of average T2FR.
Table 2.
Two-level hierarchical linear model (see eqs. 1–3) of the firing of striatal head movement related neurons during T2 (after cocaine injection): final estimation of between-neuron fixed effects
S.E. are robust. All the reported values in the table have been rounded. Grand mean dose = 9.68; grand mean average T1FR = 5.02.
| Fixed Effect | Parameter | Coefficient | S.E. | ta |
|---|---|---|---|---|
| Results for within-neuron intercept (β0j)b | ||||
| Between-neuron intercept (overall mean β0j value) | γ00 | 6.1108 | 0.6110 | 10.00*** |
| Dose | γ01 | −0.1101 | 0.0505 | −2.18* |
| Average T1FR | γ02 | 1.0537 | 0.1732 | 6.08*** |
| Dose2 | γ03 | −0.0242 | 0.0079 | −3.06** |
| Dose·average T1FR | γ04 | −0.0322 | 0.0107 | −3.00** |
| Dose2·average T1FR | γ05 | −0.0045 | 0.0020 | −2.23* |
| Results for within-neuron slope (β1j)c | ||||
| Between-neuron intercept (overall mean β1j value) | γ10 | 0.1742 | 0.0671 | 2.60* |
| Dose | γ11 | −0.0105 | 0.0040 | −2.66* |
| Average T1FR | γ12 | 0.0173 | 0.0073 | 2.38* |
| Dose2 | γ13 | −0.0008 | 0.0008 | −0.98 |
| Dose·average T1FR | γ14 | −0.0002 | 0.0003 | −0.66 |
| Dose2·average T1FR | γ15 | −0.0002 | 0.0001 | −1.86 |
Approximate df = 102.
Ninety-three percent of the variance of β0j explained.
Thirteen percent of the variance of β1j explained.
p < 0.05;
p < 0.01;
p < 0.001.
Table 3.
Two-level hierarchical linear model (see eqs. 1–3) of the firing of striatal head movement related neurons during T2 (after cocaine injection): final estimation of between-neuron variance components
The χ2 test statistics reported below are based on 101 of 108 neuronal units that had sufficient data for computation. All the reported values in the table have been rounded.
| Random Effect | Parameter | S.D. | Variance Component | χ2a |
|---|---|---|---|---|
| Within-neuron intercept (β0j) | μ0 | 2.1451 | 4.6015 | 3179.94*** |
| Within-neuron slope (β1j) | μ1 | 0.2014 | 0.0406 | 301.93*** |
| Within-neuron effect | r | 2.9450 | 8.6728 |
df = 95.
p < 0.001.
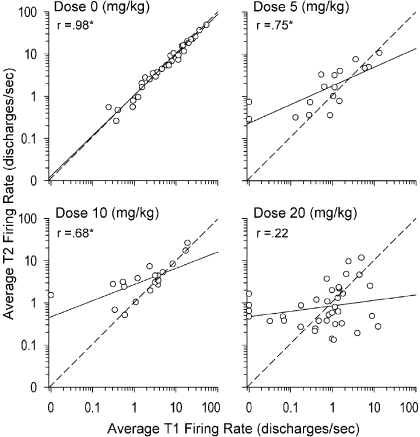
The OLS regression of each neuron's EB estimated average T2FR on its raw average T1FR is plotted in Fig. 3, with one scatter plot per dose, along with its Pearson's correlation value. Each scatter plot also shows a diagonal reference line that represents the hypothetical line of no change, that is, the OLS regression line that would be expected if the average FR across neurons maintained a high level of stability across T1 and T2. The parameters of the regression lines for each dose (with the S.E. in parentheses), along with the 95% confidence intervals (CI) for the slope, are for Dose 0, intercept = 0.026 (0.029), slope = 0.958 (0.031), 95% CI = (0.895, 1.021), R2 = 0.968; for Dose 5, intercept = 0.250 (0.086), slope = 0.443 (0.101), 95% CI = (0.226, 0.660), R2 = 0.563; for Dose 10, intercept = 0.435 (0.073), slope = 0.384 (0.098), 95% CI = (0.177, 0.591), R2 = 0.461; and for Dose 20, intercept = −0.071 (0.081), slope = 0.129 (0.096), 95% CI = (−0.066, 0.324), R2 = 0.048. The correlations and linear regressions show that, at Dose 0, there was a strong stability between the average T1FR and the average T2FR across neurons, whereas this stability progressively decreased for Doses 5 and 10 and was absent at Dose 20. Moreover, the 95% CIs showed that the slope of the regression line for Dose 0 was not statistically different from 1.00 but that the slope of the regression line for Dose 20 was not statistically different from 0.
Fig. 3.
Graphic representation of most salient results of the hierarchical linear model (text and Tables 2 and 3). EB estimates derived from HLM (see eqs. 1–3) of the average T2FR and raw average T1FR plotted for each dose of cocaine using scatter plots. Each dot represents one neuron. For ease of interpreting changes across the spectrum of slow- and fast-firing neurons, x- and y-axes (T1FR and T2FR, respectively) were both log10-transformed. Solid line represents OLS regression line for log10-transformed FR. The r values are the correlations (∗p < 0.05) between the log10-transformed average T1FR and T2FR for each dose. Dashed line represents the line of no change. Dots above the dashed line: average T2FR of the neuron increased from its average T1FR; dots below the dashed line: average T2FR of the neuron decreased from its average T1FR. Progressive clockwise rotation of regression lines (solid lines) with increasing dose of cocaine was indicated in HLM by significant between-neuron negative interaction parameters associated with dose · average T1FR (γ04 = −0.0322) and dose2 · average T1FR (γ05 = −0.0045) for within-neuron intercept parameter (β0), i.e., average T2 FR.
This dose-dependent pattern of “clockwise” rotation of the between-neuron OLS regression lines shows a FR-dependent effect of cocaine. That is, at Doses 5 and 10, the increases in average T2FR of neurons with lower average T1FR were the main determinant of the clockwise rotation of their regression lines. At Dose 20, both the increases of average T2FR of neurons with lower average T1FR and the decreases of average T2FR of neurons with higher average T1FR together determined the greater clockwise rotation of the regression line. Several neurons that exhibited little or no firing in T1 exhibited substantial increases in FR in the presence of 20 mg of cocaine. The fastest-firing neurons at this dose exhibited the strongest suppressions of FR in T2 (see below). Seven neurons at Doses 5, 10, and 20, but none at Dose 0, exhibited an average FR of 0 in T1 but robust elevations in their average FR in T2.
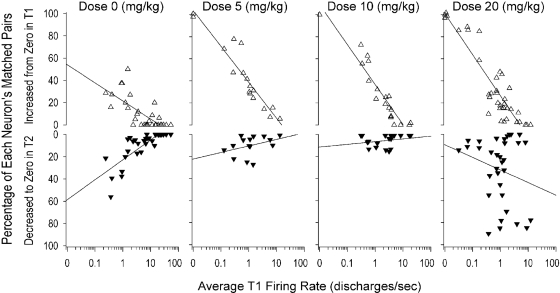
For most neurons, some matched pairs exhibited a FR of 0 discharges/s in either (but not both) T1 or T2. In such cases, the complete lack of firing in one period suggests that the neuron was not responding to inputs that were driving it in the other period; thus, it was worthwhile to selectively examine these matched pairs. The percentage of matched pairs that exhibited 0 FR in either T1 or T2 for each neuron was computed (Fig. 4). For neurons whose matched pairs exhibited a FR of 0 in T1, i.e., elevation from 0, there was a statistically significant interaction between dose and average T1FR, F(3,100) = 10.69, p < 0.0001, in the percentage of matched pairs per neuron that increased from 0 FR in T1 to a nonzero FR in T2. Post hoc Dunnett's tests showed that for an average T1FR level of 0.58 discharges/s (i.e., slower-firing neurons), all three drug doses produced significantly more elevations than Dose 0: Dose 5, t(100) = 4.83, p < 0.0001; Dose 10, t(100) = 3.17, p < 0.01; Dose 20, t(100) = 4.51, p < 0.0001. In contrast, post hoc Dunnett's tests showed that for an average T1FR level of 5.89 discharge/s (faster-firing neurons), none of the three drug doses produced a significantly different number of elevations than Dose 0 (p > 0.05). Figure 4 also illustrates the interaction between dose and average T1FR in the percentage of matched pairs per neuron that decreased from nonzero FR in T1 to 0 FR in T2, F(3,100) = 3.18, p < 0.05. Post hoc Dunnett's tests showed one significant change: at an average T1FR level of 5.89 discharges/s (faster-firing neurons), a significantly higher number of suppressions at Dose 20 than at Dose 0, t(100) = 3.69, p < 0.001. Thus, consistent with the dose-dependent, clockwise rotation, Doses 5, 10, and 20 caused significant elevations of zero T1FR in neurons with slower average T1FR. In contrast, for faster-firing neurons, the high dose alone caused complete suppression to zero of a significant percentage of FRs that were nonzero in T1.
Fig. 4.
Each triangle represents one neuron. Top row, open upward pointing triangles: percentage of each neuron's matched pairs that increased from zero FR in time 1 (T1FR = 0) to nonzero value in T2 (T2FR ≠ 0) Bottom row, solid downward pointing triangles: percentage of each neuron's matched pairs that decreased from a nonzero T1FR value to a zero T2FR value (y-axis inverted to visually suggest the decrease to zero FR). In each row, each neuron's percentage is regressed onto its average T1FR (log10-transformed) at each dose of cocaine. Solid line, regression line for each scatter plot. For ease of readability, overlapping triangles with an average T1FR of 0 were jittered.
Dose-dependent effect of cocaine on within-neuron slopes of the HLM.
For the within-neuron level slope (β1j) portion of the model, the T1–T2 HLM revealed a significant main effect of dose, γ11 = −0.0105, t(102) = −2.65, p < 0.001, and a significant main effect of average T1FR, γ12 = 0.0173, t(102) = 2.37, p < 0.01 (Tables 2 and 3), but no significant interaction between the two. As dose increased, the results for the γ11 parameter indicated that, in regressing T2FR on T1FR of individual neurons (Fig. 5), there was a significant clockwise shift for within-neuron slopes, i.e., they became progressively more negative with increasing dose. The means (with S.D. in parentheses) of the EB slope estimates for Doses 0 through 20 were 0.22 (0.18), 0.19 (0.12), 0.14 (0.15), and −0.02 (0.05), respectively. In contrast, as average T1FR increased, the results for the γ12 parameter indicated that there was a tendency for within-neuron slopes to become more positive, i.e., to shift counterclockwise. Note that the latter was not a drug effect.
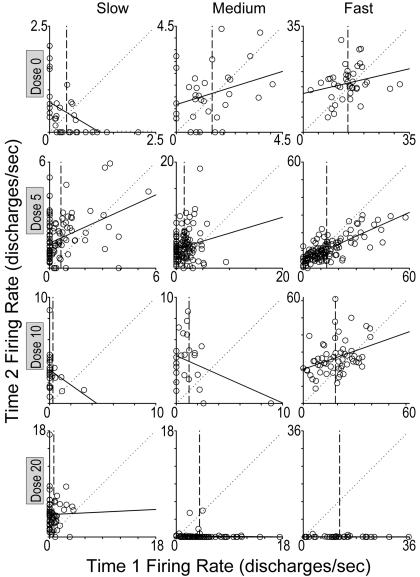
Fig. 5.
Scatter plots regressing T2FR (y-axis) on T1FR (x-axis) of each matched pair of 12 individual neurons. Each graph represents one neuron, and each dot represents one of its matched pairs. Neurons that exhibited average T1FR that were representative of slow-, medium-, or fast-firing neurons are illustrated for each dose of cocaine (milligrams per kilogram) shown at left of each row. Lengths of both axes for each scatter plot were customized to accommodate the T1FR and T2FR for each neuron and set to be equivalent to each other. Solid line, OLS regression line for each neuron, which corresponds to the within-neuron portion of the HLM (see equation 1). Dotted diagonal reference line, line of no change, i.e., regression line that would be expected if the T1FR and T2FR of the neuron's matched pairs maintained perfect stability across T1 and T2. Vertical line corresponds to the average T1FR of the neuron, which was used as the centering point for T1FR in equation 1 of the HLM. Value on y-axis for point of intersection of vertical reference line and regression line corresponds to the value of the centered intercept in equation 1, which is the average T2FR of the neuron.
The proportion of variance of the within-neuron slope explained by all the between-neuron predictors was 0.13. The proportion of variance of the T2FR values of the matched pairs at the within-neuron level explained exclusively by T1FR was 0.10, which indicates that for an individual striatal neuron during a particular movement, T1FR is a weak predictor of T2FR when the movement recurs (in T2). Thus, in the HLM, the proportion of variance in average T2FR explained by average T1FR was much greater than the proportion of variance in individual T2FR values of matched pairs explained by individual T1FR (0.69 > 0.10). Visual inspection of individual scatter plots (T2FR regressed on T1FR) of all 108 neurons revealed a corresponding high degree of scatter. OLS regression analysis of matched pairs within individual neurons showed low R2 values that did not differ across dose, F(3,103) = 0.510, p > 0.05. The overall average R2 value of all the neurons was 0.13. Figure 5 presents scatter plots of 12 representative neurons using all the matched pairs of each neuron.
Statistically, the low R2 values and the low proportion of variance of the within-neuron slope explained by the average T1FR for all the neurons indicate that any given matched pair's T1FR is a poor predictor of its T2FR. This implies that for a given matched pair of an individual neuron, its T2FR could lie anywhere within the range of the T2FR of all the matched pairs of that neuron, regardless of the T1FR of the matched pair. This variability occurred despite moderate-to-strong relationships of most neurons to movement parameters apex, distance, duration, and velocity (Pederson et al., 1997), which were “held constant” within each matched pair. Test-retest variability in striatal responsiveness to somatosensory stimulation is similarly high (Prokopenko et al., 2004).
FR-dependent effect of cocaine on the within-neuron slopes of the HLM.
Graphic analysis of scatter plots of all the neurons revealed additional striking patterns. Relative to the line of no change (slope = 1.0), the slope of the regression line for 99 of 101 neurons exhibited a clockwise rotation that appeared to differ across dose, in agreement with the above main effect of dose (γ11) in the within-neuron level slope (β1j) portion of the T1–T2 HLM (excluding seven neurons that exhibited a uniform constant T1FR = 0). To represent this pattern without presenting scatter plots of all 101 neurons, one average standardized measure of change {[T2FR/(T1FR + T2FR)] − 0.5} was computed for each neuron's high FR matched pairs, and another for its low FR matched pairs. These were plotted against average T1FR and connected by a line (one line for each neuron; Fig. 7). A repeated-measures nested ANCOVA on the standardized measures of change revealed a significant three-way interaction of dose, matched pair FR level (high versus low), and average T1FR, F(3,3933) = 5.60, p < 0.001. Among neurons along the entire range of FR in T1 at Dose 0, the clockwise shift of individual neurons' regression lines (Fig. 5) reflected a pattern of “regression to the mean.” That is, on average, low T1FR matched pairs showed higher FRs in T2, and high T1FR matched pairs showed lower FRs in T2. This pattern is illustrated by matched pairs of representative neurons at Dose 0 (Fig. 5). The three-way interaction indicates that FR at Doses 5, 10, and 20 significantly deviated from this pattern of regression to the mean.
Fig. 7.
Average standardized value of change in FR {average[(T2FR/{T1FR + T2FR}) − 0.5]} for low versus high T1FR matched pairs regressed onto average T1FR for all four doses of cocaine. Solid dots represent average standardized value of change for matched pairs in which T1FR ≤ average T1FR of their respective neuron. Open circles represent the average standardized value of change for matched pairs in which T1FR > average T1FR of their respective neuron. Solid lines connect average standardized values of change of low and high T1FR matched pairs from the same neuron. Note: seven neurons exhibited only a low FR (solid dot) because for them, average T1FR = 0; thus, all their matched pairs were assigned to the low FR category. Dashed horizontal line represents line of no change; y-axis values >0 represent increases above T1FR; y-axis values <0 represent decreases below T1FR.
At Dose 5, post hoc Dunnett's tests revealed that low FR matched pairs showed a significant increase in FR relative to Dose 0 at an average T1FR level of 0.1 discharges/s, t(3933) = 5.08, p < 0.001, and also at an average T1FR level of 1 discharge/s, t(3933) = 4.90, p < 0.001. At Dose 10, post hoc Dunnett's tests revealed that low FR matched pairs showed a significant increase in FR relative to Dose 0 at an average T1FR level of 0.1 discharges/s, t(3933) = 3.86, p < 0.001, and at an average T1FR level of 1 discharge/s, t(3933) = 3.93, p < 0.001. Thus, at Doses 5 and 10, the pattern underlying clockwise rotations was significantly different from the pattern of regression to the mean observed at Dose 0 for slower and moderately firing neurons (approximate average T1FR < ≈1 discharge/s). Figure 5 shows that at Doses 5 and 10 this enhancing effect on FR of low T1FR matched pairs for slow-firing neurons was absent for fast-firing neurons (approximate average T1FR >1 discharge/s). These effects are further illustrated by raster/PETHs of a representative neuron with slow-to-medium T1FR at Dose 10 (Fig. 6).
At Dose 20, a different pattern was observed: post hoc Dunnett's tests revealed a suppressive effect at an average T1FR level of 10 discharges/s (i.e., faster-firing neurons; Fig. 7) for both low T1FR matched pairs, t(3933) = −5.46, p < 0.0001, and high T1FR matched pairs, t(3933) = −3.72, p < 0.001. These effects are further illustrated by the matched pairs of representative medium- and fast-firing neurons at Dose 20 (Fig. 5). Consistent elevations of FR at Dose 20 were observed for only the lowest T1FR (Figs. 5 and 7).
A test of variance on the standardized change values in FR using repeated-measures nested ANCOVA revealed a significant three-way interaction of dose, matched pair FR level, and average T1FR, t(3933) = 8.39, p < 0.0001. Post hoc Dunnett's tests showed that at Dose 10, there was a significant decrease in variability for low FR matched pairs relative to Dose 0 at an average T1FR level of 0.1 discharges/s, t(3933) = −2.75, p < 0.05, and at an average T1FR level of 1 discharge/s, t(3933) = −2.61, p < 0.01. This reflects somewhat uniform increases of low FR of slower-firing neurons. At Dose 20, low FR matched pairs showed a significant increase in variability relative to Dose 0 at an average T1FR level of 0.1 discharges/s, t(3933) = 2.68, p < 0.05, and at an average T1FR level of 1 discharge/s, t(3933) = 2.94, p < 0.01. High FR matched pairs at Dose 20 showed an increase in variability relative to Dose 0 at an average T1FR level of 1 discharge/s, t(3933) = 2.67, p < 0.05, and at an average T1FR level of 10 discharges/s, t(3933) = 2.48, p < 0.05. That is, low FR matched pairs of slow- and moderate-firing neurons and high FR matched pairs of moderate- to fast-firing neurons exhibited a wide range of increases and decreases in T2 at Dose 20.
Analyses of Reversal.
To assess whether the above between-neuron (average FR) changes reversed in T3, 2587 matched pairs between T1 and T3 were obtained from 63 neurons that exhibited stable neural activity in T3 at Dose 5 (n = 13), 10 (n = 15), and 20 (n = 35) (T3 data were not collected for Dose 0). The median number of matched pairs (with the minimum and maximum values in parentheses) obtained per neuron in the reduced dataset used for the reversal analyses for Doses 5, 10, and 20 were 49 (3, 121), 18 (7, 95), and 35 (1, 122), respectively. An HLM on these matched pairs revealed that there was neither significant main effect of dose (dose or dose2) nor significant interaction of dose and the average T1FR (dose · MT1FR or dose2 · MT1FR) on the average T3FR of individual neurons (data not shown). This indicates that the neurons' average T3FR was not significantly different across the three drug doses. The HLM did reveal that the main effect of average T1FR was significant, γ02 = 0.62, t(57) = 3.12, p < 0.01, which indicated that the average T1FR irrespective of dose was a significant predictor of average T3FR.
The OLS regression line (data not shown) of each neuron's EB estimated average T3FR on its raw average T1FR (both on a log-transformed scale) was computed. The parameters of the regression lines for each dose (with the S.E. in parentheses), along with the 95% CIs for the slope, were for Dose 5, intercept = 0.296 (0.147), slope = 0.453 (0.161), 95% CI = (0.094, 0.812), R2 = 0.418; for Dose 10, intercept = 0.091 (0.126), slope = 0.695 (0.210), 95% CI = (0.237, 1.153), R2 = 0.457; and for Dose 20, intercept = −0.227 (0.124), slope = 0.304 (0.145), 95% CI = (0.009, 0.599), R2 = 0.118. The linear OLS regression parameters, along with the R2 values, indicated that Doses 5 and 10 maintained essentially the same levels of stability between T1 and T3 compared with the stability between T1 and T2. The linear regressions also indicated that Dose 20 exhibited an increase in stability between T1 and T3 compared with the (lack of) stability between T1 and T2. Moreover, the 95% CIs showed that the slope of the regression line of T3FR on T1FR for Dose 10 was not statistically different from 1.00 and that the slope of the regression line for Dose 20 of T3FR on T1FR was statistically different from 0. Thus, several of the effects that led to a significant dose-dependent pattern of clockwise rotation of between-neuron regression lines observed from T1 to T2 were absent in T3, having returned toward predrug levels.
Within the set of matched pairs that exhibited 0 FR in T1 and nonzero FR in T2, i.e., elevation from 0 FR, reversal for the low FR neurons (low FR defined as having an average T1FR less than 1.48) was assessed by testing whether average T3FR was less than average T2FR in Doses 5, 10, and 20. The LS means of T2FR and T3FR (with S.E. in parentheses) for the low FR neurons followed by the results of the nested t test were Dose 5, 1.28 (0.35) and 1.85 (0.59), t(348) = −1.69, p > 0.05; Dose 10, 2.47 (0.41) and 1.08 (0.59), t(284) = 3.61, p < 0.001; and Dose 20, 1.12 (0.20) and 0.65 (0.22), t(586) = 4.68, p < 0.0001. Thus, in T3, significant reversal (decrease) of T2 elevations from zero T1FR for slower neurons was observed at Doses 10 and 20 but not at Dose 5; however, Dose 5 did not significantly further increase its FR in T3.
Within the set of matched pairs that exhibited nonzero FR in T1 and 0 FR in T2, i.e., suppression to 0 FR, reversal was assessed for the fast FR neurons (fast FR defined as having an average T1FR ≥ 1.48) at Dose 20 by testing whether T3FR was greater than 0. The LS mean of T3FR (with the S.E. in parentheses) for the fast FR neurons was 0.42 (0.33). A one-sample nested, t test, t(208) = 1.27, p > 0.21 indicated that, although it was not significantly different from 0, FR was increasing (reversing) in T3, relative to T2 in which all the values of these matched pairs were zero.
To assess whether the above, within-neuron (slope) changes reversed, the 2587 matched pairs from 63 neurons between T1 and T3 described previously were used. An HLM on these matched pairs revealed that the two main effects that were significant for the within-neuron slope portion of the T1–T2 HLM, i.e., dose and average T1FR, were not significant for T1–T3 (data not shown). For comparison purposes, the HLM of T2FR regressed on T1FR was rerun on the dataset used for the T3 recovery analyses. The means (with S.D. in parentheses) of the EB estimated within-neuron slopes from the T1–T2 HLM model for Doses 5, 10, and 20 were 0.30 (0.08), 0.18 (0.09), and −0.01 (0.02), respectively. The means (with S.D. in parentheses) of EB estimated within-neuron slopes from the T1–T3 HLM model for Doses 5, 10, and 20 were 0.35 (0.19), 0.06 (0.42), and 0.05 (0.57), respectively. Across all the drug doses, 69.8% of neurons exhibited an increased value of T1–T3 within-neuron slope relative to the T1–T2 within-neuron slope (i.e., counterclockwise rotation), indicating a return toward predrug FR in T3.
Discussion
Assessments of changes in FR in the lateral striatum were made simultaneously with behavioral effects known to involve pharmacological actions of stimulants in the lateral striatum (Creese and Iversen, 1974; Kelley and Delfs, 1994). Stereotypy occurred selectively at the high dose. Repetitive, purposeless movements took the form of repeated, criterion head movements that earned reinforcers while animals continued head bobbing without stopping to drink. Neither the increased percentage of long-distance movements at low doses (5 and 10 mg/kg) nor the decrease at the high dose (20 mg/kg) (Pederson et al., 1997) were observed at Dose 0. In T3, all the behavioral effects recovered, and most changes in FR either recovered or reversed the direction of change (full recovery may require more time in some cases; Ma et al., 1999). Although results discussed below could reflect cocaine's actions at sites afferent to striatum, e.g., cortex (Rutter et al., 2005), such influences likely had less effect on striatal firing than did receptor-mediated actions of cocaine or DA directly in the striatum.
We addressed the question: what changes in firing of striatal neurons specifically related to movements induced by systemic stimulants occur when the drug is stimulating those movements? Eliminating sensorimotor variability appears to be a key difference from earlier studies that showed decreased, increased, or unchanged striatal FR. All were observed in the present study, but they conformed to a significant pattern revealed by a second key difference from earlier studies, i.e., to sort cocaine-induced changes in FR as a function of predrug FR.
At the between-neuron level (i.e., sample of all the neurons), cocaine disrupted the stability of average striatal FR in a dose- and rate-dependent manner. Stability of firing was shown at Dose 0, at which every neuron exhibited no change in average FR from T1 to T2. These results indicate that the FR of a striatal neuron is centered around a particular value that exhibits remarkable consistency across time. With increasing dose, stability decreased as the regression lines between average T2FR and T1FR shifted in a systematic clockwise manner. At low to moderate doses (5 and 10 mg/kg), we observed exclusively increases in average FR in T2. These increases were FR-dependent, i.e., were observed only for slower-firing neurons. At the highest dose (20 mg/kg), both increases and decreases in average FR were observed in T2, but these changes exhibited a nonrandom, FR-dependent pattern as well. As observed at the lower doses, slower-firing neurons increased their average FR, but unique to the high dose was the substantially decreased FR of faster-firing neurons.
At the within-neuron level, matched pairs within each neuron exhibited regression to the mean at Dose 0, in that FRs that were low in T1 tended to be higher in T2, and FRs that were high in T1 tended to be lower in T2. For an individual neuron, without any drug injected, a matched pair's T2FR cannot be predicted from its T1FR, despite virtually identical movements (see Prokopenko et al., 2004). Although scatter plots of individual neurons' matched pairs depicting T2FR regressed on T1FR exhibited a high degree of scatter, they revealed striking effects of cocaine that also were found to depend on the predrug FR of the matched pair, as well as on dose. For slower-firing neurons at Doses 5, 10, and 20, elevations were observed only on movements associated with low or zero T1FR, whereas their high T1FR were unaffected. Thus, the elevation of average FR of slower-firing neurons across doses (described above) was in fact accounted for by selective elevations of their matched pairs with low T1FR. Faster-firing neurons were unaffected by low doses but were affected only by the high dose. Their medium and high predrug FRs were reduced—in some neurons strongly suppressed—by the high dose. Thus, at the high dose, the neurons that were most suppressed were not those whose T1FR were already near zero, but rather were the faster-firing neurons, for which even the highest predrug FRs were suppressed to zero. Also characteristic of the high dose was a significant increase in variability among neurons, i.e., a large increase in individual differences with respect to T1–T2 FR changes. Furthermore, at Dose 20, cocaine's effect was not uniform among low and high FR of individual neurons. Slower-firing neurons exhibited wide variations in magnitude and direction of the T1–T2 FR change for their low T1FR matched pairs. Faster-firing neurons exhibited equally wide variations in magnitude and direction of the T1–T2 FR change for their high T1FR matched pairs. This contrasts with the singular, more orderly elevation of low FR of slower-firing neurons observed at the low and moderate doses.
All the neurons corresponded to type IIb, medium spiny, projection neurons (Kimura et al., 1990), comprising 95% of the striatal population. Striatal variations in FR likely reflect differing degrees of excitatory corticostriatal synaptic input (Stern et al., 1997), as recognized by hypotheses that DA transmission suppresses weak and enhances strong excitatory striatal inputs (Nicola et al., 2000; O'Donnell, 2003). Inconsistent with these hypotheses, all three doses elevated average FR of slow-firing neurons, and the high dose suppressed average FR of faster-firing neurons (see Tang et al., 2008). Moreover, within individual neurons, cocaine dose dependently enhanced low and reduced high T1FR, which likely reflects a given neuron's responses to weak versus strong synaptic inputs, respectively. Especially convincing was the enhancement of FRs that were zero in T1, presumably representing subthreshold afferent inputs. These inputs from sensorimotor cortex were likely of similar strength in T1 and T2, given that the movement in T1 was matched in T2, but under the influence of cocaine became capable of inducing discharges. In fact, seven neurons exhibiting only 0 FR in T1 were induced to fire by cocaine. At the high dose, in which enhancement of subthreshold inputs was prominent, moderate and high FR matched pairs of faster-firing neurons, which almost certainly reflect strong excitatory cortical inputs, were, with few exceptions, strongly suppressed. Together with the opposite pattern caused by reduced striatal DA transmission (Prokopenko et al., 2004), our findings contradict theories proposing that DA agonists filter out weak and enhance strong corticostriatal inputs. In the absence of attempts by proponents of these hypotheses to test them using behaviorally relevant corticostriatal signals in awake animals, our studies are among few models that have done so.
An important question that addresses previous electrophysiological findings regarding stimulant effects on striatal firing regards the behavioral relevance for the present high-dose effects. The acute induction of stereotypy requires high brain levels of cocaine, which can be achieved by high intraperitoneal or intravenous single doses (Tella, 1994). Lower levels of intravenous cocaine do not initially induce stereotypy (Tella, 1994) when self-administered (Lecca et al., 2007), but with repeated intravenous self-administration of these same levels, stereotypy is acquired (Lecca et al., 2007). This suggests that brain levels of cocaine that animals self-administer may be lower than those capable of acutely inducing stereotypy, as induced only by the high dose in the present study. Indeed, striatal levels of cocaine and DA generally have been reported to be lower during intravenous maintenance than after acute intraperitoneal injections of 15 to 30 mg/kg (Nicolaysen et al., 1988; Pettit and Justice, 1991; Weiss et al., 1992; Stuber et al., 2005). The present, high dose increases in variability and suppressions of striatal FR—as well as the suppressions characteristic of many previous studies of DA agonist effects on striatal FR—may occur only at high agonist levels. Thus, our exclusive increases in FR obtained at low and moderate doses that stimulate nonstereotyped movements indicate that only one, simple change in movement-related FR of lateral striatal neurons should be expected at these doses of acutely administered cocaine: elevation of low FR of slower-firing neurons (West et al., 1997). This is consistent with the fact that increased FR is the only direction of change during normal movement reported in the literature for lateral striatal neurons phasically related to movement. Given that focused stereotypy can be acquired with repeated intravenous self-administration of cocaine, it might be hypothesized that this would be accompanied by recruitment of the present high-dose pattern of elevated low FR and reduced high FR in movement-related striatal activity, but this prediction is complicated by the virtual elimination of movement-related striatal activity with repeated training (Carelli et al., 1997; Tang et al., 2007). No data are yet available regarding the firing of these neurons during repeated cocaine self-administration.
The elevation of movement-related FR of lateral striatal neurons by behaviorally relevant, acute doses of cocaine (see Tang et al., 2008) is consistent with recent suggestions of medial-to-lateral functional partitioning in striatum (Ikemoto et al., 2005) and our own observations of medial-to-lateral transitions in MSN activity. Neurons in the accumbens core, compared with neurons in the more medial shell, exhibit stronger relations to instrumental responses, less responsiveness to auditory discriminative stimuli, and greater prevalence of elevated FR during cocaine self-administration (Ghitza et al., 2006). Further laterally than the core, in the lateral or sensorimotor striatum, MSNs exhibit robust, unconditioned relations to activity of body parts (Carelli and West, 1991), little responsiveness to visual (Kimura, 1990) or auditory (Carelli et al., 1997) discriminative stimuli, and only elevations of FR in the presence of low to moderate levels of cocaine (Tang et al., 2008; present study). How these functional differences fit with the laterally spiraling connections of striatal structures via mesencephalic and thalamocortical loops (Haber et al., 2000) will be important in understanding basal ganglia function in health and disease.
Acknowledgments
We thank our departed colleague, Dr. Volodimir Prokopenko, for intellectual and technical support. We thank Drs. Erich Labouvie and Greg Camilli for statistical advice, David Root for comments on previous versions of this manuscript and for technical assistance, and Linda King, Sisi Ma, and Ed Kuang for technical assistance.
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA004551, DA006886].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.158253
- DA
- dopamine
- DLS
- dorsolateral striatum
- MSN
- medium spiny neuron
- FR
- firing rate
- HLM
- hierarchical linear model
- ANOVA
- analysis of variance
- LED
- light-emitting diode
- T1
- precocaine time epoch
- T2
- cocaine time epoch
- T3
- recovery time epoch
- PETH
- perievent time histogram
- 3D
- three-dimensional
- EB
- empirical Bayesian
- OLS
- ordinary least squares
- ANCOVA
- analysis of covariance
- LS
- least square
- CI
- confidence interval.
References
- Abdi H. (2007) O'Brien test for homogeneity of variance, in Encyclopedia of Measurement and Statistics, Volume 2 (Salkind N. ed) pp 701–704, Sage Publications, Thousand Oaks, CA: [Google Scholar]
- Carelli RM, West MO. (1991) Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol 309:231–249 [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wolske M, West MO. (1997) Loss of lever press-related firing of rat striatal forelimb neurons after repeated sessions in a lever pressing task. J Neurosci 17:1804–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Iversen SD. (1974) The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia 39:345–357 [DOI] [PubMed] [Google Scholar]
- Crutcher MD, DeLong MR. (1984) Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res 53:244–258 [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Jr, Kilbey MM. (1975) Amphetamine stereotypy: the influence of environmental factors and prepotent behavioral patterns on its topography and development. Biol Psychiatry 10:3–16 [PubMed] [Google Scholar]
- Fog R, Pakkenberg H. (1971) Behavioral effects of dopamine and p-hydroxyamphetamine injected into corpus striatum of rats. Exp Neurol 31:75–86 [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. (1984) Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 13:1189–1215 [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO, Fabbricatore AT. (2006) Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self-administration. Neuroscience 137:1075–1085 [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. (2000) Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20:2369–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haracz JL, Tschanz JT, Wang Z, Griffith KE, Rebec GV. (1998) Amphetamine effects on striatal neurons: implications for models of dopamine function. Neurosci Biobehav Rev 22:613–622 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. (2005) The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle valid? J Neurosci 25:5061–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. (1994) Excitatory amino acid receptors mediate the orofacial stereotypy elicited by dopaminergic stimulation of the ventrolateral striatum. Neuroscience 60:85–95 [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. (1975) Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res 94:507–522 [DOI] [PubMed] [Google Scholar]
- Kimura M. (1990) Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol 63:1277–1296 [DOI] [PubMed] [Google Scholar]
- Kimura M, Kato M, Shimazaki H. (1990) Physiological properties of projection neurons in the monkey striatum to the globus pallidus. Exp Brain Res 82:672–676 [DOI] [PubMed] [Google Scholar]
- Künzle H.(1975) Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88:195–209 [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. (2007) Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 191:653–667 [DOI] [PubMed] [Google Scholar]
- Lyon M, Robbins TW. (1975) The action of central nervous system stimulant drugs: a general theory concerning amphetamine effects, in Current Developments in Psychopharmacology, Volume 2 (Essman W, Valzelli L. eds) pp 79–163, Spectrum Publications, New York: [Google Scholar]
- Ma F, Falk JL, Lau CE. (1999) Within-subject variability in cocaine pharmacokinetics and pharmacodynamics after intraperitoneal compared with intravenous cocaine administration. Exp Clin Psychopharmacol 7:3–12 [DOI] [PubMed] [Google Scholar]
- Ma S, Root DH, Tang C, Pawlak AP, Barker DJ, West MO. (2009) Absence of cue-evoked firing in dorsolateral striatum neurons during the formation and expression of habitual behavior. Soc Neurosci Abstr Program 567:Poster 13 Annual Meeting of the Society for Neuroscience; 2009 Oct 17–21; Chicago, IL Society for Neuroscience, Washington, DC: [Google Scholar]
- McGeorge AJ, Faull RL. (1989) The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29:503–537 [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. (2000) Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23:185–215 [DOI] [PubMed] [Google Scholar]
- Nicolaysen LC, Pan HT, Justice JB., Jr (1988) Extracellular cocaine and dopamine concentrations are linearly related in rat striatum. Brain Res 456:317–323 [DOI] [PubMed] [Google Scholar]
- O'Donnell P. (2003) Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17:429–435 [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2005) The Rat Brain in Stereotaxic Coordinates, 5th ed, Academic Press, San Diego, CA: [Google Scholar]
- Pederson CL, Wolske M, Peoples LL, West MO. (1997) Firing rate dependent effect of cocaine on single neurons of the rat lateral striatum. Brain Res 760:261–265 [DOI] [PubMed] [Google Scholar]
- Peoples LL, West MO. (1996) Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci 16:3459–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr (1991) Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res 539:94–102 [DOI] [PubMed] [Google Scholar]
- Porrino LJ. (1993) Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology (Berl) 112:343–351 [DOI] [PubMed] [Google Scholar]
- Prokopenko VF, Pawlak AP, West MO. (2004) Fluctuations in somatosensory responsiveness and baseline firing rates of neurons in the lateral striatum of freely moving rats: effects of intranigral apomorphine. Neuroscience 125:1077–1082 [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. (2002) Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd ed, Sage Publications, Thousand Oaks, CA: [Google Scholar]
- Rutter JJ, Devilbiss DM, Waterhouse BD. (2005) Effects of systemically administered cocaine on sensory responses to peri-threshold vibrissae stimulation: individual cells, ensemble activity, and animal behaviour. Eur J Neurosci 22:3205–3216 [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. (1997) Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol 77:1697–1715 [DOI] [PubMed] [Google Scholar]
- Stevens JP. (2002) Applied Multivariate Statistics for the Social Sciences, 4th ed, Lawrence Erlbaum Associates, Mahwah, NJ: [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. (2005) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology 30:853–863 [DOI] [PubMed] [Google Scholar]
- Tang C, Mittler T, Duke DC, Zhu Y, Pawlak AP, West MO. (2008) Dose- and rate-dependent effects of cocaine on striatal firing related to licking. J Pharmacol Exp Ther 324:701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Pawlak AP, Prokopenko V, West MO. (2007) Changes in activity of the striatum during formation of a motor habit. Eur J Neurocsi 25:1212–1227 [DOI] [PubMed] [Google Scholar]
- Tella SR. (1994) Differential blockade of chronic versus acute effects of intravenous cocaine by dopamine receptor antagonists. Pharmacol Biochem Behav 48:151–159 [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. (1979) Effects of D-amphetamine on striatal unit activity and behavior in freely moving cats. Neuropharmacology 18:735–738 [DOI] [PubMed] [Google Scholar]
- Weiss F, Hurd YL, Ungerstedt U, Markou A, Plotsky PM, Koob GF. (1992) Neurochemical correlates of cocaine and ethanol self-administration. Ann N Y Acad Sci 654:220–241 [DOI] [PubMed] [Google Scholar]
- West MO, Peoples LL, Michael AJ, Chapin JK, Woodward DJ. (1997) Low-dose amphetamine elevates movement-related firing of rat striatal neurons. Brain Res 745:331–335 [DOI] [PubMed] [Google Scholar]