Abstract
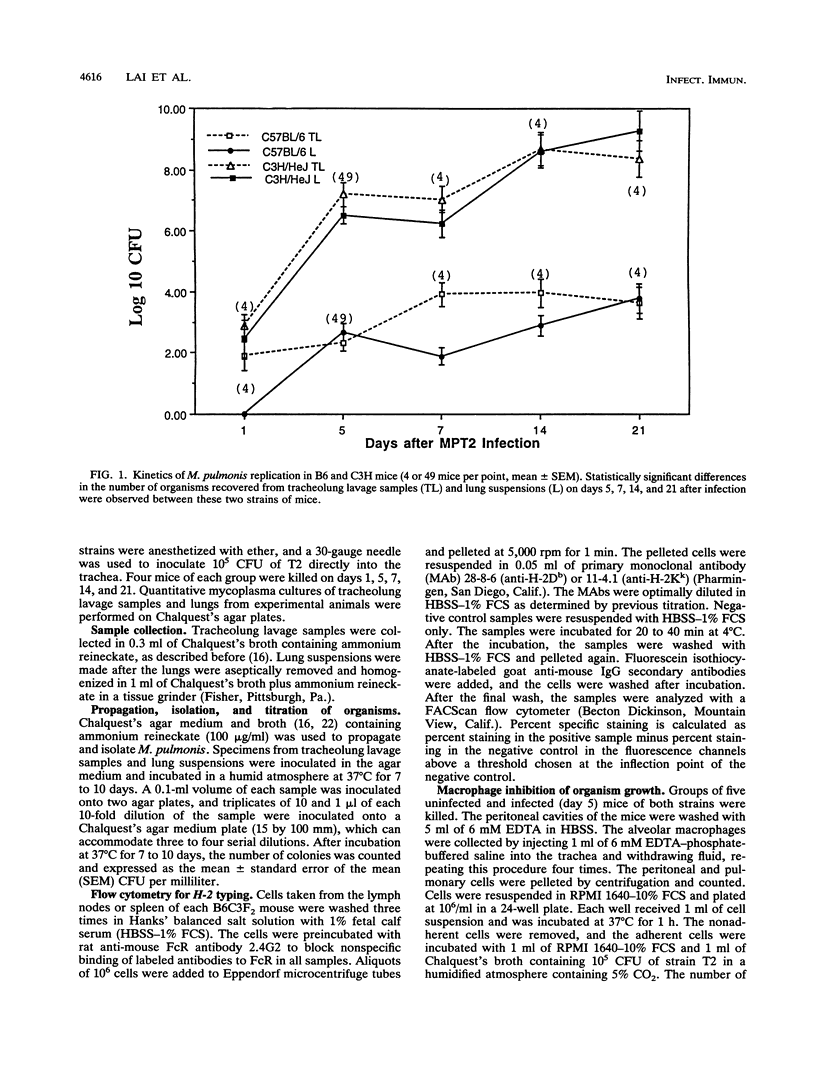
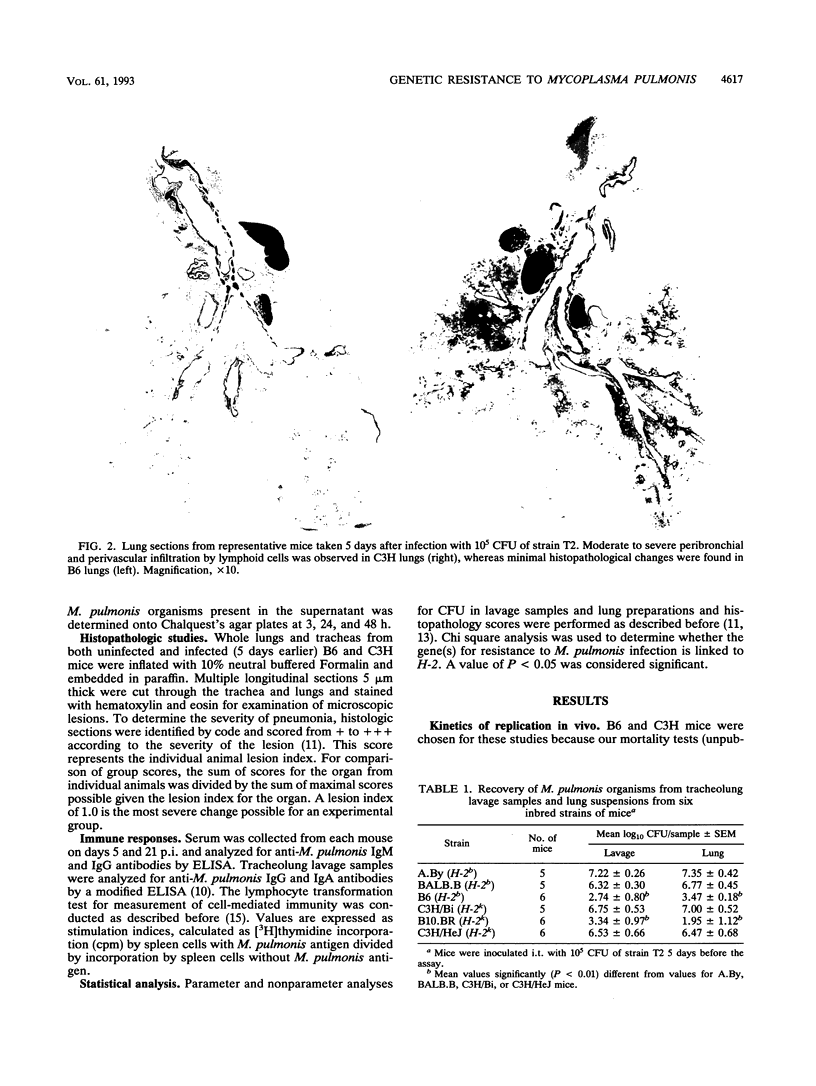
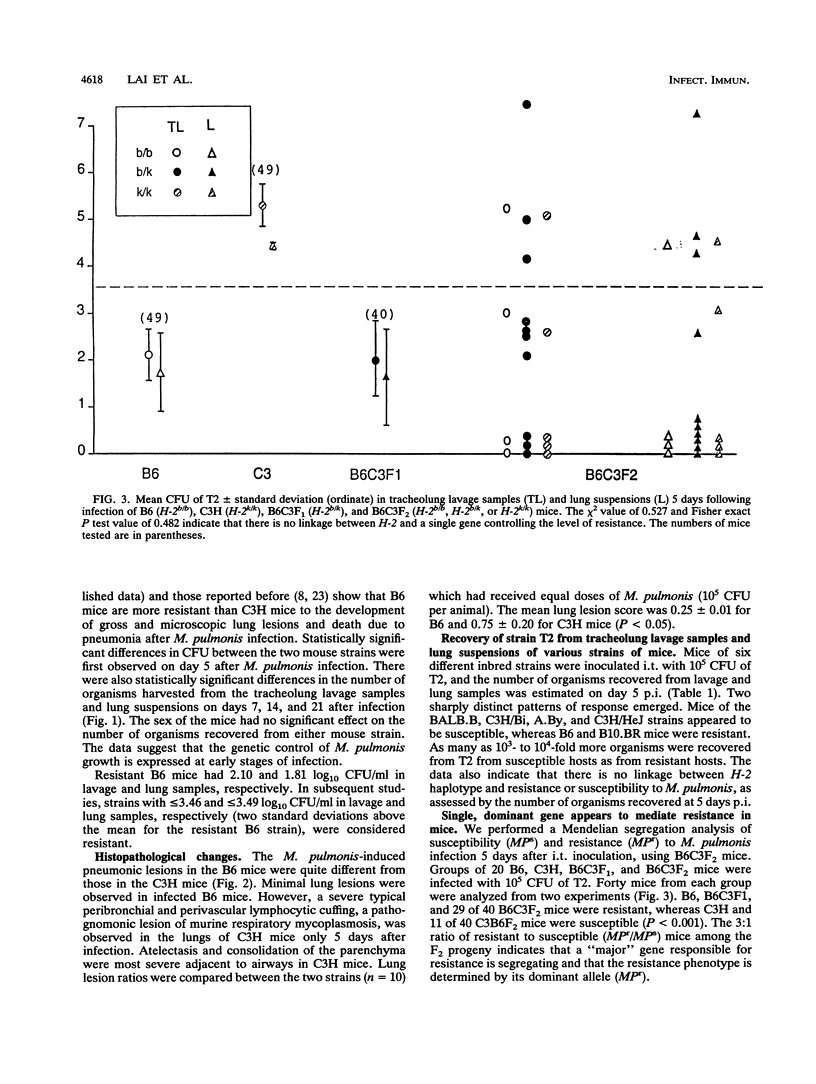
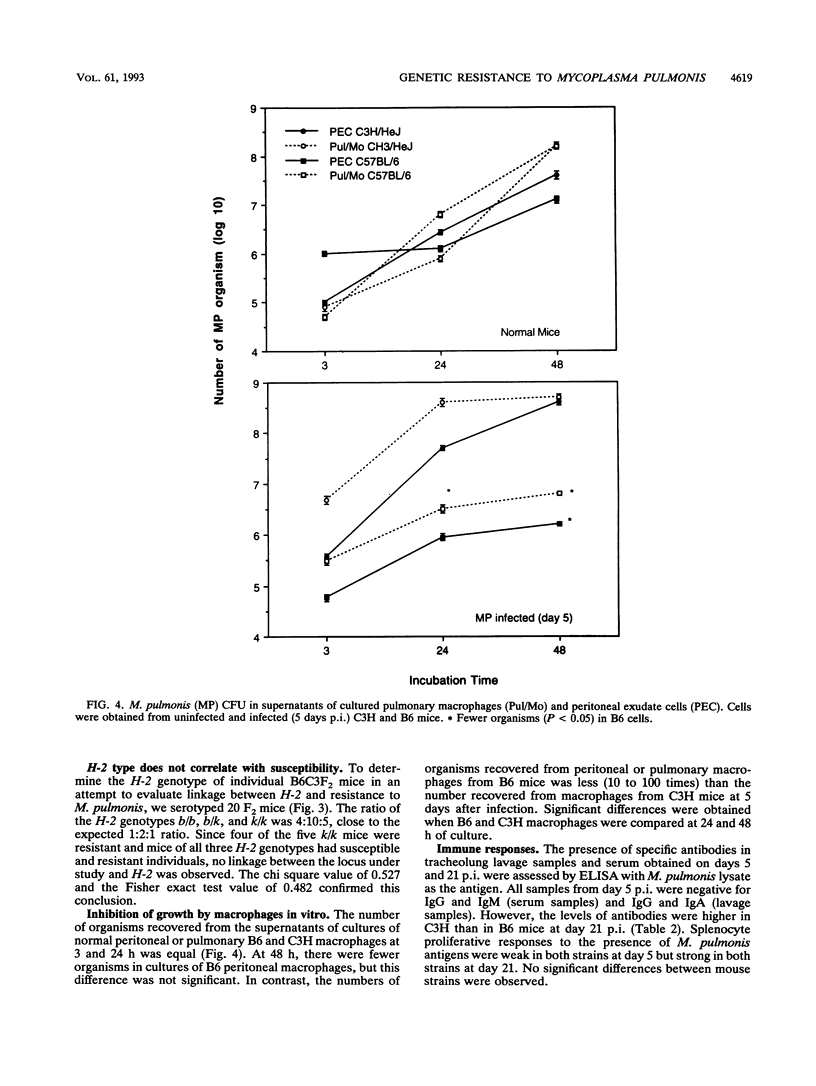
The differences in susceptibility of various inbred strains of mice to a highly pathogenic strain of Mycoplasma pulmonis CT (T2) has been known for some time. We assessed the genetic control of resistance to T2 infection. Tracheolung lavage samples and lungs of mice were assessed for T2 organisms after intratracheal injection of T2. We found that H-2b (C57BL/6 (B6) and H-2k B10.BR mice were resistant, whereas H-2b A.By, H-2k C3H/Bi, H-2k C3H/HeJ (C3H), and H-2b BALB.B mice were susceptible. We also typed individual B6C3F2 mice for H-2 and for resistance to T2 and observed that resistance to T2 infections is controlled by a single dominant gene not linked to H-2. Histologic examination revealed severe lung lesions typical of M. pulmonis infections in susceptible C3H mice, in contrast to minimal lung lesions in resistant B6 mice. No significant titers of local or systemic antimycoplasma antibodies were detected in either resistant or susceptible mice at 5 days postinfection. Macrophages taken from uninfected B6 or C3H mice failed to inhibit growth of T2 in vitro. However, macrophages from B6 mice did inhibit growth of T2 much better than C3H macrophages when harvested on day 5 of infection. Thus, there is an association between activation of macrophage bactericidal function and genetic resistance to growth of T2 organisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguila H. N., Pakes S. P., Lai W. C., Lu Y. S. The effect of transportation stress on splenic natural killer cell activity in C57BL/6J mice. Lab Anim Sci. 1988 Apr;38(2):148–151. [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Born W., Harbeck R., O'Brien R. L. Possible links between immune system and stress response: the role of gamma delta T lymphocytes. Semin Immunol. 1991 Jan;3(1):43–48. [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Parker R. F., White H., Dziedzic D., Taylor G., Davidson M. K., Cox N. R., Cassell G. H. Strain differences in susceptibility to murine respiratory mycoplasmosis in C57BL/6 and C3H/HeN mice. Infect Immun. 1985 Dec;50(3):647–654. doi: 10.1128/iai.50.3.647-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy J. M., Levey-Leblond E., Le Prevost C. Immunopathology of mouse hepatitis virus type 3mii. effect of immunosuppression in resistant mice. J Immunol. 1975 Jan;114(1 Pt 1):226–230. [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W. C., Bennett M., Lu Y. S., Pakes S. P. Biological evaluation of Mycoplasma pulmonis temperature-sensitive mutants for use as possible rodent vaccines. Infect Immun. 1990 Jul;58(7):2289–2296. doi: 10.1128/iai.58.7.2289-2296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W. C., Bennett M., Pakes S. P., Kumar V., Steutermann D., Owusu I., Mikhael A. Resistance to Mycoplasma pulmonis mediated by activated natural killer cells. J Infect Dis. 1990 Jun;161(6):1269–1275. doi: 10.1093/infdis/161.6.1269. [DOI] [PubMed] [Google Scholar]
- Lai W. C., Bennett M., Pakes S. P., Murphree S. S. Potential subunit vaccine against Mycoplasma pulmonis purified by a protective monoclonal antibody. Vaccine. 1991 Mar;9(3):177–184. doi: 10.1016/0264-410x(91)90150-5. [DOI] [PubMed] [Google Scholar]
- Lai W. C., Pakes S. P., Lu Y. S., Brayton C. F. Mycoplasma pulmonis infection augments natural killer cell activity in mice. Lab Anim Sci. 1987 Jun;37(3):299–303. [PubMed] [Google Scholar]
- Lai W. C., Pakes S. P., Owusu I., Wang S. Mycoplasma pulmonis depresses humoral and cell-mediated responses in mice. Lab Anim Sci. 1989 Jan;39(1):11–15. [PubMed] [Google Scholar]
- Lai W. C., Pakes S. P., Stefanu C., Lu Y. S. Comparison of Chalquest and Hayflick media, with and without ammonium reineckate, for isolating Mycoplasma pulmonis from rats. J Clin Microbiol. 1986 May;23(5):817–821. doi: 10.1128/jcm.23.5.817-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Formal S. B. Effect of silica on the innate resistance of inbred mice to Salmonella typhimurium infection. Infect Immun. 1979 Aug;25(2):513–520. doi: 10.1128/iai.25.2.513-520.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson N. O., Kerr K. M., Campbell A. Control of infectious synovitis. 12. Preparation of an agglutination test antigen. Avian Dis. 1963 Aug;7(3):310–317. [PubMed] [Google Scholar]
- Parker R. F., Davis J. K., Blalock D. K., Thorp R. B., Simecka J. W., Cassell G. H. Pulmonary clearance of Mycoplasma pulmonis in C57BL/6N and C3H/HeN mice. Infect Immun. 1987 Nov;55(11):2631–2635. doi: 10.1128/iai.55.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Saito M., Nakagawa M., Muto T., Imaizumi K. Strain difference of mouse in susceptibility to Mycoplasma pulmonis infection. Nihon Juigaku Zasshi. 1978 Dec;40(6):697–705. doi: 10.1292/jvms1939.40.697. [DOI] [PubMed] [Google Scholar]
- Schurr E., Skamene E., Forget A., Gros P. Linkage analysis of the Bcg gene on mouse chromosome 1. Identification of a tightly linked marker. J Immunol. 1989 Jun 15;142(12):4507–4513. [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]
- Taylor G., Howard C. J. Protection of mice against Mycoplasma pulmonis infection using purified mouse immunoglobulins: comparison between protective effect and biological properties of immunoglobulin classes. Immunology. 1981 Jul;43(3):519–525. [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Taylor-Robinson D. Effects of active and passive immunization on Mycoplasma pulmonis-induced pneumonia in mice. Immunology. 1976 May;30(5):611–618. [PMC free article] [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]