FIG. 4.
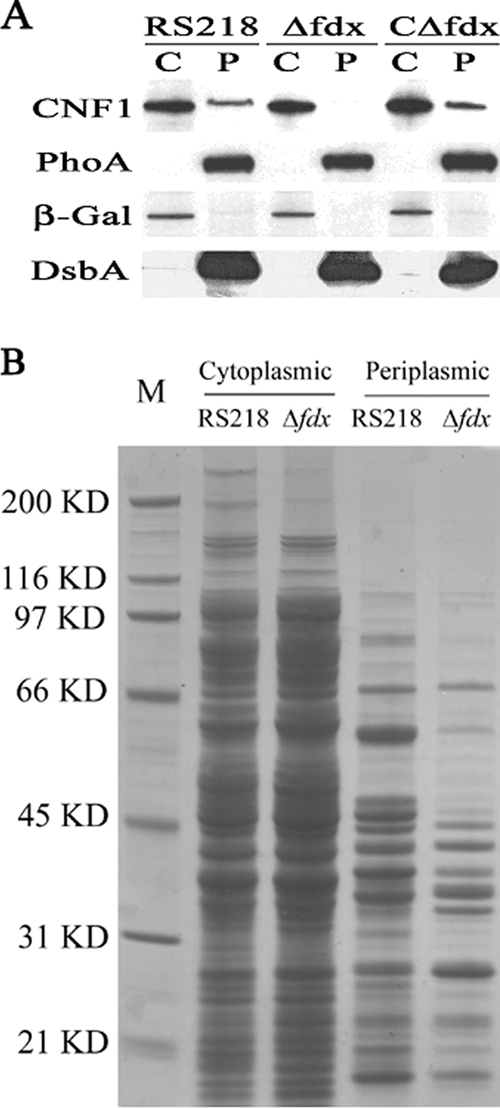
(A) CNF1 subcellular localization and secretion in E. coli RS218. Western blot analysis was carried out with the cytoplasmic (C) and periplasmic (P) fractions from RS218, Δfdx, and CΔfdx (with 0.1% arabinose to promote the transcription of complemented fdx gene). The amount of cytoplasmic protein loaded was 40 μg, and the amount of periplasmic protein loaded was equal to the total periplasmic protein that was collected from 3 ×109 bacteria (the number of bacteria was estimated from the optical density at 620 nm [OD620]). CNF1, PhoA, β-Gal, and DsbA were detected by their respective specific antibodies, as described in Materials and Methods. (B) SDS-PAGE analysis of the protein profile in the cytoplasmic and periplasmic fractions prepared from RS218 and Δfdx as indicated in the figure. M, molecular marker; size positions are indicated along with the masses on the left. The amount of cytoplasmic protein loaded was 15 μg. The loaded periplasmic protein was equal to the total periplasmic protein that was collected from 1010 bacteria cells (the number of bacteria was estimated from the OD620).
