Abstract
A chimeric gene, MSP-Fu24, was constructed by genetically coupling immunodominant, conserved regions of the two leading malaria vaccine candidates, Plasmodium falciparum merozoite surface protein 1 (C-terminal 19-kDa region [PfMSP-119]) and merozoite surface protein 3 (11-kDa conserved region [PfMSP-311]). The recombinant MSP-Fu24 protein was produced in Escherichia coli cells and purified to homogeneity by a two-step purification process with a yield of ∼30 mg/liter. Analyses of conformational properties of MSP-Fu24 using PfMSP-119-specific monoclonal antibody showed that the conformational epitopes of PfMSP-119 that may be critical for the generation of the antiparasitic immune response remained intact in the fusion protein. Recombinant MSP-Fu24 was highly immunogenic in mice and in rabbits when formulated with two different human-compatible adjuvants and induced an immune response against both PfMSP-119 and PfMSP-311. Purified anti-MSP-Fu24 antibodies showed invasion inhibition of P. falciparum 3D7 and FCR parasites, and this effect was found to be dependent on antibodies specific for the PfMSP-119 component. The protective potential of MSP-Fu24 was demonstrated by in vitro parasite growth inhibition using an antibody-dependent cell inhibition (ADCI) assay with anti-MSP-Fu24 antibodies. Overall, the antiparasitic activity was mediated by a combination of growth-inhibitory antibodies generated by both the PfMSP-119 and PfMSP-311 components of the MSP-Fu24 protein. The antiparasitic activities elicited by anti-MSP-Fu24 antibodies were comparable to those elicited by antibodies generated with immunization with a physical mixture of two component antigens, PfMSP-119 and PfMSP-311. The fusion protein induces a protective immune response with human-compatible adjuvants and may form a part of a multicomponent malaria vaccine.
Malaria is among the major parasitic diseases in tropical and subtropical countries. With as many as 300 to 500 million new cases each year, malaria accounts for the death of over 2 million people globally each year, and most are children (41). Among the four species of Plasmodium that infect humans, the most threatening is Plasmodium falciparum. The extensive spread of drug-resistant P. falciparum strains as well as the insecticide-resistant mosquito necessitates the development of a malaria vaccine on an urgent basis. Collectively, the major objective of the ongoing vaccine effort in this field is to develop a multistage, multivalent vaccine against P. falciparum (34).
The blood-stage cycle of the parasite is responsible for malaria pathogenesis. Intervention at this stage of the parasite's development through vaccination is likely to reduce malaria-related clinical symptoms. As a major interface between host and pathogen, the merozoite surface is an obvious target for the development of a malaria vaccine. A number of potential vaccine candidate antigens identified so far are located on or associated with the surface of the merozoite or in apical organelles. These include merozoite surface protein 1 (MSP-1), MSP-2, MSP-3, MSP-4, MSP-5, MSP-8, RAP1/2, AMA-1, and EBA-175, which are implicated in the process of merozoite invasion of the erythrocyte (23).
MSP-1 is one of the most extensively studied proteins of P. falciparum (18). It is synthesized as a ∼200-kDa precursor and then processed in two steps: the primary processing step produces a complex of four fragments that are present on the merozoite surface, and the secondary processing step at invasion results in the shedding of the complex from the surface, except for the C-terminal 19-kDa domain (MSP-119), which remains anchored to the parasite surface by a glycosylphosphatidylinositol (GPI) moiety (2). The C-terminal 19-kDa fragment of MSP-1 is well conserved among P. falciparum isolates and contains two epidermal growth factor (EGF)-like domains that play a role in merozoite invasion. Substantial data from studies with P. falciparum MSP-1 and in vivo immunization studies of mice with Plasmodium yoelii and Plasmodium chabaudi indicate that the protective immune responses are directed against the C-terminal 19-kDa domain (10, 12, 15, 20, 27, 35). The inhibition of MSP-1 processing by conformation-specific antibodies (Abs) was previously proposed to be one of the possible mechanism for the inhibition of merozoite invasion (1).
Another merozoite surface protein, MSP-3, was also shown to be the target of the protective immune responses in humans (29). The PfMSP-3 protein contains three blocks of four tandem heptad repeats based on the AXXAXX motif at the N terminus, a glutamic acid-rich domain, and a putative leucine zipper sequence at the C terminus (25). Although a clear surface localization of PfMSP-3 is known, it lacks any transmembrane domain or glycosylphosphatidylinositol (GPI) anchor site (24, 25) and is therefore considered to be loosely associated with the merozoite surface by interactions with other merozoite surface proteins. PfMSP-3 was identified as a candidate vaccine antigen by an antibody-dependent cellular inhibition (ADCI) assay using human immune sera (28). The potential of PfMSP-3 as a vaccine candidate was further illustrated by ADCI using mice antibodies and was further confirmed by the suppression of P. falciparum growth in an immunocompromised mouse after the passive transfer of human antibodies purified on MSP-3 peptides together with human monocytes (28, 40, 42). The immunization of Aotus and Saimiri monkeys with recombinant PfMSP-3 or its fragments provided protection against parasite challenge (6, 16). A 70-amino-acid-long conserved domain of PfMSP-3, referred to here as the PfMSP-311 region, was identified as the target of protective antibodies in human immune responses (40). The presence of high titers of cytophilic antibodies, IgG3, against this conserved region of MSP-3 has been correlated with protection against the parasite. In addition, immunization of humans with a synthetic peptide corresponding to this region was previously shown to induce antiparasitic antibodies that suppress parasite growth in an ADCI assay (11).
It is generally believed that a combination vaccine for malaria is likely to be more effective than vaccines based on a single antigen, and attempts are being made to develop a malaria vaccine by using a mixture of more than one antigen or by combining immunologically relevant proteins of the target antigens as fusion proteins (31, 43, 45). In the present study, we have constructed a fusion chimera (MSP-Fu24) consisting of PfMSP-119 and PfMSP-311 and produced the corresponding recombinant MSP-Fu24 protein in Escherichia coli cells. The two individual components, PfMSP-119 and PfMSP-311, were also expressed and purified separately; the immunological properties of MSP-Fu24 were compared with a physical mixture of the two individual components. MSP-Fu24 retained the native conformation of the PfMSP-119 component and was highly immunogenic in small animals. The anti-MSP-Fu24 antibodies inhibited parasite invasion into host red blood cells (RBCs) and also inhibited parasite growth in a monocyte-dependent manner, suggesting the potential of the fusion protein as a malaria vaccine candidate.
MATERIALS AND METHODS
Construction of plasmids expressing PfMSP-311, PfMSP-119, and MSP-Fu24.
To design a synthetic gene corresponding to the C-terminal 19-kDa fragment of PfMSP-1, the amino acid sequence of PfMSP-119 corresponding to residues 1526 to 1619 of the P. falciparum Welcome strain (GenBank accession no. P04933) was back-translated to the nucleotide sequence based on the E. coli codon frequency table (available at http://www.kazusa.or.jp/codon). This synthetic gene was used as a template to amplify PfMSP-119 with the NcoI-XhoI site with the following primer set: forward primer 5′-GTG ACA CCA TGG GTA ACA TTT CTC AGC ATC AGT G-3′ and reverse primer 5′-GCC CTC GAG TTA GTG GTG GTG GTG GTG GTG GGA ACT GCA GAA AAT ACC ATC-3′. The amplified product was cloned into the NcoI and XhoI sites of pET28a(+), a kanamycin-based vector (Novagen), in frame with the coding sequence of the 6×His tag at its C terminus, to obtain the pET28a-PfMSP-119 construct.
The conserved 11-kDa fragment of PfMSP-3 corresponding to 70 amino acids (163 to 230 amino acids) was amplified from a PfMSP-3 synthetic gene with the following set of primers: forward primer 5′-GGC GGC CAT GGC AAA GAA TGC TTA CGA AAA GGC C-3′ and reverse primer 5′-GGC CTC GAG TTA GTG GTG GTG GTG GTG GTG GTC GTT TTC CTT AGA GAT GTT TTC-3′. The amplified product was cloned into the NcoI and XhoI sites of pET28a+, in frame with the coding sequence of the 6×His tag at its C terminus, to obtain the pET28a-PfMSP-311 construct.
To generate a fusion construct consisting of PfMSP-311 and PfMSP-119, the 11-kDa fragment of PfMSP-3 was amplified again with different sets of primers: forward primer 5′-GGC GGC CAT GGC AAA GAA TGC TTA CGA AAA GGC C-3′ and reverse primer 5′-GCC GCC CAT GGC GTC GTT TTC CTT AGA GAT GTT TTC-3′. The amplified PCR product was purified and digested with NcoI. The excised fragment was cloned into the NcoI site of pET28a-PfMSP-119 to generate the pET28a-MSP-Fu24 construct.
All constructs were sequenced from both ends to confirm the orientation and sequence of the inserts and transformed into E. coli BLR(DE3) cells (Novagen) for the expression of recombinant proteins with 6×His tags.
Expression and purification of recombinant proteins.
E. coli BLR(DE3) cells containing recombinant plasmids pET28a-PfMSP-119, pET28a-PfMSP-311, and pET28a-MSP-Fu24 were grown in Luria broth containing kanamycin (30 μg/ml) at 37°C until an optical density at 600 nm (OD600) of 0.6 to 0.7 was reached. The expressions of the respective recombinant proteins were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C, and the expressed proteins were analyzed and localized by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting of the soluble and insoluble fractions of the E. coli cells after disruption.
For the purification of recombinant MSP-Fu24 and PfMSP-119, the E. coli cell pellets from the respective 6-liter shake flask cultures were washed with phosphate-buffed saline (PBS) and resuspended in lysis buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 10 mM imidazole, 1% Triton X-100, 25 mg liter−1 lysozyme, 5 mM benzamidine HCl). The bacterial cells were lysed on ice by sonication, and the lysate was centrifuged at 12,000 rpm for 45 min at 4°C. The clarified supernatant was loaded onto a column containing precharged streamline chelating matrix (GE Healthcare). The column was subsequently washed with 10 column volumes of equilibration buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 10 mM imidazole), followed by 10 column volumes each of wash buffer 1 (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 40 mM imidazole) and wash buffer 2 (20 mM Tris-HCl [pH 8.0], 10 mM NaCl, 40 mM imidazole). Bound protein was eluted with a linear gradient of imidazole (40 mM to 1 M) in 20 mM Tris-10 mM NaCl (pH 8.0) buffer. The eluted fractions were analyzed by SDS-PAGE, and fractions containing the recombinant protein were pooled. The pooled protein was further purified by anion-exchange chromatography on a column of Q-Sepharose resin (GE Healthcare) equilibrated with equilibration buffer (20 mM Tris-HCl [pH 8.0], 10 mM NaCl). The bound proteins were eluted with a linear gradient of NaCl (10 mM to 1 M) in Tris-HCl buffer (pH 8.0). Eluates were analyzed by SDS-PAGE, fractions containing a single protein band of MSP-Fu24 or PfMSP-119 were pooled, and the protein concentration was determined by a bicinchoninic acid assay (BCA).
For the purification of PfMSP-311, the cell pellet from the 6-liter shake flask culture was lysed by sonication, and the inclusion bodies (IBs) were collected by centrifugation at 12,000 rpm. The IB pellet was resuspended in solubilization buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 10 mM imidazole, 8 M urea) and kept for 12 to 15 h under stirring at room temperature (RT). The solubilized IBs were centrifuged at 12,000 rpm for 45 min at room temperature, and clarified supernatant was loaded onto a column containing precharged streamline chelating matrix (GE Healthcare). The column was sequentially washed with 10 column volumes of equilibration buffer (20 mM Tris-HCl [pH 8.0], 500 mM NaCl, 10 mM imidazole, 8 M urea), wash buffer 1 containing 8 M urea, and wash buffer 2 containing 8 M urea. The bound protein was eluted by a linear gradient of imidazole (40 mM to 500 mM) in elution buffer (20 mM Tris-HCl [pH 8.0], 10 mM NaCl, 8 M urea). The fractions were analyzed by SDS-PAGE, and those fractions containing the PfMSP-311 protein were pooled and dialyzed sequentially against 6 M, 4 M, and 2 M urea in dialysis buffer (20 mM Tris-HCl [pH 8.0]), followed by a final change in 20 mM Tris-10 mM NaCl (pH 8.0). Dialyzed PfMSP-311 was further purified by anion-exchange chromatography by using the Q Sepharose matrix, as mentioned above.
The homogeneity of purified MSP-Fu24, PfMSP-311, and PfMSP-119 was assessed by SDS-PAGE under reducing and nonreducing conditions, on an analytical gel permeation chromatography column, and by reverse-phase chromatography on an analytical C8 column (Supelcosil; 5 by 4.9 cm, 5 μm). Endotoxin contents in the protein samples were estimated by using a Limulus amebocyte lysate (LAL) gel clot assay (Charles River Endosafe), and host cell protein contamination was estimated by immunoblotting as well as by enzyme-linked immunosorbent assay (ELISA) using anti-E. coli antibodies (Cygnus Technologies). The reactivity of the recombinant proteins with monoclonal and polyclonal antibodies was analyzed by immunoblotting according to standard protocols. Briefly, the recombinant proteins were separated by SDS-PAGE under reducing or nonreducing conditions and blotted onto a nitrocellulose membrane, followed by the blocking of the membrane using 5% nonfat milk in PBS (pH 7.4). The blots were sequentially incubated with the respective monoclonal or polyclonal antibodies in PBS (pH 7.4) containing 0.5% milk and 0.05% Tween 20, followed by the respective horseradish peroxidase-conjugated secondary antibody, after prior washing with PBS containing 0.05% Tween 20 (PBS-T). The protein bands were detected after developing the reaction mixture with 3,3′-diaminobenzidine tetrahydrochloride (DAB) in PBS and hydrogen peroxide (H2O2).
Immunization of mice and rabbits with recombinant antigens formulated with Freund's adjuvant (IFA), alum, and Montanide ISA720.
Three different adjuvants used in the immunization study were Freund's complete adjuvant (CFA) and Freund's incomplete adjuvant (Sigma), alum (Alhydrogel; Superfos, Denmark), and Montanide ISA 720 (Seppic Inc., France). Vaccine formulations were prepared with the individual antigen or their mixture according to the manufacturers' instructions. Groups of five BALB/c mice 4 to 6 weeks of age were immunized intramuscularly with 25 μg of the individual antigens (MSP-Fu24, PfMSP-311, or PfMSP-119) or a mixture of PfMSP-311 and PfMSP-119 (25 μg each) in each of the adjuvant formulations. Control groups received only PBS mixed with adjuvants. Immunized animals were given booster doses with the respective formulations on days 28 and 56 and were bled on days 0, 14, 42, 55, and 70 after the first immunization. Four groups of two New Zealand White rabbits were immunized intradermally with 200 μg of MSP-Fu24 or with a mixture of PfMSP-311 and PfMSP-119 (200 μg each) emulsified with either Montanide ISA720 or alum. Two booster immunizations were carried out intramuscularly on day 28 and day 56 by using the respective adjuvant formulations, and mice were bled on day 70 after the first immunization. The sera obtained were used for immunoassay and IgG purification. For the duration of this study, mice and rabbits were housed and used strictly in accordance with the guidelines set by the National Institutes of Health in 1985, and the study was approved by the institutional animal ethics committee of the International Centre for Genetic Engineering and Biotechnology (ICGEB).
IgG purification from rabbit and mouse sera.
Total IgG was purified from sera obtained from immunized groups of mice and rabbits as well as from the control groups by using a protein G-Sepharose column (Pharmacia) according to the manufacturer's recommendations. Briefly, a serum sample, after equal dilution with binding buffer (20 mM sodium phosphate buffer [pH 7.0]), was loaded onto a protein G-Sepharose column preequilibrated with binding buffer. The column was washed with 10 column volumes of the binding buffer. The bound IgG was eluted with 0.2 M glycine-HCl (pH 3.0), and eluted fractions were analyzed by SDS-PAGE. The fractions containing purified IgGs were pooled and dialyzed against PBS.
Human immune sera from a region where malaria is endemic.
Forty-two human sera were collected from healthy individuals residing in an area where P. falciparum is endemic (Orissa, India); these individuals were without any blood-stage infection at the time of collection and had no recent history of any malaria infection. Consent from these individuals and approval from the Human Volunteers Research Ethical Committee of the International Centre for Genetic Engineering and Biotechnology were obtained prior to the study. Serum samples were also collected from individuals who had no known history of malaria and had never visited an area where malaria is endemic.
ELISA and competitive ELISA.
Antibody responses in mice as well as the reactivity of human sera and monoclonal antibodies (MAbs) (12.10, 2F10, and 5.2) with the respective antigens were evaluated by ELISA. All ELISAs were carried out using a 100-μl reaction mixture volume. Briefly, 96-well microplates (Dynatech) were coated with 100 ng of the antigens (MSP-Fu24, PfMSP-311, or PfMSP-119; antigen concentration, 1 μg/ml) diluted in 0.06 M carbonate-bicarbonate buffer (pH 9.6) per well. Plates were washed with 1× PBS containing 0.05% Tween 20 (PBS-T), and the wells were blocked with 5% low-fat milk in PBS (pH 7.2) for 1 h at room temperature. Antigen-coated wells were sequentially incubated with serial dilutions of immune sera from mouse or rabbit and then with enzyme-labeled secondary antibody (horseradish peroxidase-labeled anti-mouse or anti-human immunoglobulin [IgG]). In between these incubations, the plates were washed with PBS-T. The enzyme reaction mixture was developed with o-phenylenediamine dihydrochloride-H2O2 in a citrate phosphate buffer (pH 5.0), the reaction was stopped with 8 M H2SO4, and the OD was recorded at 490 nm by use of a microplate reader (Molecular Devices). An OD cutoff of 0.1 (mean plus 2 standard deviations [SDs]) was selected for antibody titer determinations. To coat the reduced/denatured protein, SDS and β-mercaptoethanol were added to the protein solution to final concentrations of 1% (vol/vol) and 5% (vol/vol), respectively, kept in boiling water for 5 min, and coated into 96-well microplates at a concentration of 100 ng/well.
To detect the reactivities of MSP-Fu24, PfMSp-119, and PfMSP-311 with anti-PfMSP-1 conformation-specific monoclonal antibodies, ELISA was carried out by using MAb 12.10 and MAb 5.2 (62.5 ng/ml) under nonreducing and reducing conditions as described above. The assay for the reactivity of each of the recombinant proteins with anti-penta-His antibody (dilution, 1:1,000; Qiagen) was carried out in parallel as a positive control to ascertain equal amounts of coating of the antigens under reduced and nonreduced conditions. To detect subclasses of IgG in sera of mice immunized with MSP-Fu24 or with a mixture of PfMSP-119 and PfMSP-311, ELISA was performed as described above by using immunized mouse sera (1:10,000 dilutions); MSP-Fu24 as a capture antigen; secondary goat antibodies specific for mouse IgG1, IgG2a, IgG2b, and IgG3 (Sigma) at dilutions of 1:1,000; and horseradish peroxidase (HRP)-linked tertiary anti-goat antibodies (Sigma). The statistical significance of the results was determined by use of an unpaired Student's t test.
A competitive ELISA was carried out to assess the efficacy of purified anti-MSP-Fu24 antibodies to competitively block the binding of anti-PfMSP-119 MAbs. For this, wells of a microtiter plate were coated with 100 ng of the PfMSP-119 protein (100 μl of antigen at a concentration of 1 μg/ml) as described above. These wells were incubated with serial dilutions of the purified anti-MSP-Fu24 rabbit antibodies (2.5 μg/ml to 1.2 ng/ml) for 2 h at 37°C. After washing with PBS-T, the wells were incubated with 6.25 ng of mouse MAb 5.2 or 12.10 (100-μl solution in 1× PBS at a concentration of 62.5 ng/ml) for 2 h at 37°C. The plates were washed, and the binding of the MAbs was detected with anti-mouse IgG conjugated to horseradish peroxidase. The reactivity of PfMSP-119 with MAbs after a primary incubation with anti-MSP-Fu24 antibodies was compared with the reactivity without any incubation.
Immunofluorescence assay.
Antibodies to the recombinant proteins were tested for their reactivity with native parasite proteins by an immunofluorescence assay (IFA). The assay was performed essentially as described previously (36, 46). Briefly, multispot parasite slides were made from P. falciparum (3D7) cultures, air dried, fixed with a mixture of acetone-methanol (9:1), and then blocked in blocking buffer (10% fetal calf serum [FCS] in PBS) for 1 h at 37°C. Slides were washed and incubated for 1 h with purified IgGs against each of the antigens, appropriately diluted in blocking buffer, and kept in a sealed humid chamber for 2 h at 37°C. Slides were washed with PBS-T and subsequently incubated with anti-mouse and anti-rabbit secondary antibodies conjugated to fluorescence dye (fluorescein isothiocyanate [FITC] or Cy-3; dilution, 1:250) for 1 h at room temperature in the dark. Later, the washed slides were stained with DAPI (4′,6′-diamidino-2-phenylindole) at a concentration of 2 μg/ml, followed by two washes with PBS-T and one wash with PBS. The washed slides were then mounted with a coverslip in the presence of Antifade mounting reagent (Bio-Rad) and were viewed by using a Nikon fluorescence microscope (SE300) with a 100× oil immersion objective.
Lymphoproliferative cellular responses and cytokine analysis.
Groups of BALB/c mice were immunized with the recombinant proteins formulated in Montanide ISA720 or alum as described above. Fourteen days later, spleens of two mice from each immunized group were isolated, and single-cell suspensions were prepared from them. Cells were plated at 5 × 106 cells/ml in a final volume of 200 μl per well in 96-well flat-bottom plates containing RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 55 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 100 U of penicillin-streptomycin/ml. Cells were cultured in the presence or absence of a graded concentration of homologous antigen (4 days) and concanavalin A (2 days) in a humidified atmosphere with 5% CO2 at 37°C. Splenocytes were pulse-labeled with 1 μCi per well of [3H]thymidine (Amersham Pharmacia Biotech) per well and harvested onto glass fiber filters by using a PHD cell harvester (Cambridge Technology, Cambridge, MA) 16 h later. The [3H]thymidine incorporation was determined by β-emission liquid scintillation spectroscopy (Betaplate; Pharmacia, Sweden). The geometric mean of the counts per minute for each set of triplicate wells was calculated, and the stimulation indices were calculated as the counts per minute for the test antigen divided by the counts per minute for the control. The statistical significance of the results was determined by use of a Student's t test.
For determinations of cytokine production, 106 splenocytes were cultured in a volume of 200 μl in 96-well flat-bottom plates in the presence or absence of an antigen. Culture supernatants were collected after 60 h for gamma interferon (IFN-γ) analysis. IFN-γ was measured by using a murine cytokine immunoassay kit (Duo Set ELISA developmental system; R&D Systems, Minneapolis, MN) according to the procedure recommended by the manufacturer.
Parasite culture and invasion inhibition assay.
P. falciparum parasite strains 3D7 and FCR were cultured by using methods described previously by Trager and Jensen (44). Parasite cultures were maintained under mixed gas (5% CO2, 5% O2, and 90% N2) at 37°C in O+ erythrocytes and RPMI 1640 medium supplemented with 10% O+ human sera, 0.2% sodium bicarbonate, 0.2% glucose, and gentamicin (10 μg/ml). P. falciparum cultures were synchronized by sorbitol treatment (22).
Invasion inhibition assays were performed as described previously (36, 46). Briefly, ring-stage malaria parasites were synchronized with sorbitol lysis and allowed to mature through to the schizont stage. Hematocrit and parasitemia were adjusted to 2% and 0.5%, respectively. Purified IgG from rabbit sera (preimmune and immune) was added to the parasite culture in 96-well plates at final concentrations of 0.5, 0.25, and 0.125 mg/ml. Cultures were incubated at 37°C in a mixed-gas (5% CO2, 5% O2, and 90% N2) cabinet for 26 h to allow for schizont rupture and merozoite invasion. These assays were performed in triplicate. For microscopic analysis, smears were made from triplicate wells and stained with Giemsa stain, and the numbers of ring-stage parasites per 5,000 RBCs were determined for each well. The percent inhibition of the parasite invasion was calculated using the following formula: percent inhibition = 1 − [(percent invasion of purified IgG from immune sera)/(percent invasion of purified IgG from preimmune sera)] × 100.
ADCI assay.
The antibody-dependent cell inhibition (ADCI) assay was carried out by using purified IgG from mouse sera according to a procedure described previously by Oeuvray et al. (28). Briefly, human monocytes were purified from blood samples of healthy donors by using CD14 (monocyte/macrophage) microbeads (Miltenyi Biotech) according to the manufacturer's protocol. The study was approved by the Human Volunteers Research Ethical Committee of the International Centre for Genetic Engineering and Biotechnology prior to the study. Tightly synchronized P. falciparum 3D7 schizont-stage parasites (0.5% parasitemia and 1% hematocrit) were cocultured in a 96-well flat-bottom microculture plate with adherent human monocytes (2 × 106 monocytes) in complete medium (RPMI medium containing 10% human sera). Purified antibodies at a concentration of 50 μg/ml were added to these cultures and incubated at 37°C for 96 h in a mixed-gas environment (5% O2, 5% CO2, and 90% N2). After 48 and 72 h of growth, 50 μl of complete medium was added to each well. After 96 h of growth, parasitemia was estimated for a thin Giemsa-stained smear of RBCs from each well by counting parasitized erythrocytes in more than 10,000 erythrocytes. Control wells consisted of (i) parasite alone, (ii) parasite and control IgG (purified from naïve mouse sera), (iii) parasites and monocytes, (iv) parasites and purified IgG without monocytes, and (v) parasites, control IgG, and monocytes. The specific growth inhibition index (SGI) was calculated as follows: 1 − [(percent parasitemia with monocytes and test antibodies/percent parasitemia with test antibodies)/(percent parasitemia with monocytes and control IgG/percent parasitemia with control IgG)] × 100.
Depletion of antigen-specific antibodies and reversal of invasion/growth inhibition.
Antibodies specific to MSP-Fu24, PfMSP-119, and PfMSP-311 were depleted from total antibodies purified from rabbit/mouse sera by using the respective recombinant antigens. Briefly, purified IgGs from rabbit or mouse sera (0.25 mg/ml and 0.05 mg/ml, respectively) of the MSP-Fu24-Montanide ISA720 groups were mixed with the recombinant antigen (MSP-Fu24, PfMSP119, or PfMSP-311) at different final concentrations (0.025 mg/ml, 0.05 mg/ml, or 0.1 mg/ml for rabbit antibodies and 0.05 mg/ml for mouse antibodies) and incubated at RT for 2 h. This antigen-antibody mix was passed three times through a 1-ml Hi-Trap Ni-nitrilotriacetic acid (NTA) column (GE Healthcare) preequilibrated with 20 mM Tris-HCl (pH 8.0) to remove the protein-antibody complexes, and the flowthrough was collected, concentrated, and dialyzed against incomplete RPMI (iRPMI) medium. The respective antigen-depleted IgGs were used subsequently for invasion inhibition or ADCI assays as described above. The growth inhibition (invasion inhibition or ADCI) by depleted antibodies was compared with that by antibodies without any depletion. Recombinant P. falciparum histidine-rich protein 2 (PfHRP-2) was used for antibody depletion as a negative control for these experiments.
RESULTS
Expression of MSP-Fu24, PfMSP-119, and PfMSP-311 in E. coli and purification of recombinant proteins.
Cloned PfMSP-119 and PfMSP-311 gene sequences were genetically coupled to generate MSP-Fu24, a chimeric gene construct, in the pET28a+ vector (Fig. 1A and B), and the corresponding recombinant protein was expressed in E. coli BLR(DE3) cells. For comparison, the individual PfMSP-119 and PfMSP-311 fragments were also cloned into the pET28a+ vector and expressed in E. coli BLR(DE3) cells. The MSP-Fu24 protein was expressed as a soluble protein, and its expression level of MSP-Fu24 was two- to three-fold higher than the expression of individual PfMSP-119 and PfMSP-311 proteins under similar conditions. PfMSP-119 was also expressed as a soluble protein in the cytosolic fraction, whereas PfMSP-311 was expressed as an insoluble protein and aggregated in the inclusion bodies (IBs) of E. coli cells.
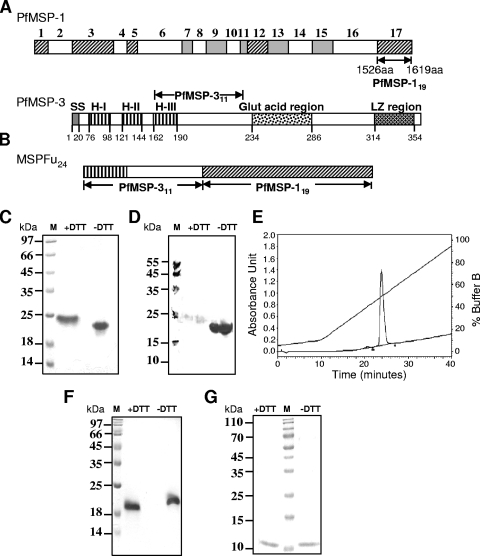
FIG. 1.
Design and expression of the chimeric protein MSP-Fu24. (A) Schematic diagram of P. falciparum MSP-1 (PfMSP-1) and PfMSP-3 showing the locations of the MSP-119 and MSP-311 regions. The conserved (hatched), semiconserved (filled), and variable (open) sequence blocks of PfMSP-1 are marked. The signal sequences (SS), heptad repeat regions (HI to HIII), glutamic acid-rich region, and leucine zipper region (LZ region) of PfMSP-3 are also marked. (B) Schematic diagram of MSP-Fu24 consisting of MSP-119 and MSP-311. (C to E) Expression and purification of recombinant MSP-Fu24. (C) Coomassie blue-stained SDS-PAGE gel showing purified recombinant MSP-Fu24 under reducing (with dithiothreitol [+DTT]) and nonreducing (−DTT) conditions. (D) Western blot analysis of MSP-Fu24 under reducing (+DTT) and nonreducing (−DTT) conditions using MAb 5.2. (E) RP-HPLC profile of purified MSP-Fu24 that eluted as a single sharp peak. (F) Coomassie blue-stained SDS-PAGE gel showing purified recombinant MSP-119 under reducing (+DTT) and nonreducing (−DTT) conditions. (G) Coomassie blue-stained SDS-PAGE gel showing purified recombinant MSP-311 under reducing (+DTT) and nonreducing (−DTT) conditions. M, molecular mass marker.
MSP-Fu24 and PfMSP-119 were purified to homogeneity from soluble fractions by a combination of metal affinity and ion-exchange chromatography. These purified recombinant proteins showed apparent mobilities of ∼24 kDa and ∼19 kDa, respectively, on SDS-PAGE gels (Fig. 1C and F). Reverse-phase high-performance liquid chromatography (RP-HPLC) and gel permeation chromatography analyses suggested that the purified MSP-Fu24 protein was >98.0% pure and was in a monomeric form (Fig. 1E). Recombinant PfMSP-311 was purified to homogeneity from the inclusion body fraction and refolded; it moved as a single band at ∼11.0 kDa under both reducing and nonreducing conditions on SDS-PAGE gels (Fig. 1G). The three purified proteins (MSP-Fu24, PfMSP-119, and PfMSP-311) eluted as monomers by gel permeation chromatography (see Fig. S1 in the supplemental material). The final yields for MSP-Fu24, PfMSP-119, and PfMSP-311 were 30.0 mg/liter, 15.0 mg/liter, and 10.0 mg/liter, respectively. The final preparations of MSP-Fu24, PfMSP-119, and PfMSP-311 contained less than 12.5, 0.25, and 5.0 endotoxin units (EU) per 25 μg of protein, respectively. Host cell proteins were not observed in any of the protein samples, as determined by an ELISA and Western blot analysis.
Immunological characterization of the fusion chimera.
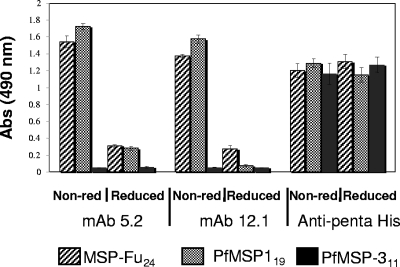
The conformational integrity of the PfMSP-119 component in MSP-Fu24 was assessed by its reactivity with conformation-specific PfMSP-119 monoclonal antibodies (MAbs 5.2 and 12.10) by immunoblotting. MSP-Fu24 also showed a strong reactivity with MAb 5.2 by immunoblotting (Fig. 1D), as in the case of PfMSP-119. ELISA was also carried out to determine the reactivities of the recombinant proteins (MSP-Fu24, PfMSP-119, and PfMSP311) with MAbs. ELISA showed that purified MSP-Fu24 and PfMSP-119 reacted strongly with monoclonal antibodies 5.2 and 12.10, which are specific for disulfide-dependent conformational epitopes of native PfMSP-119 (Fig. 2). The reactivities of MSP-Fu24 and PfMSP-119 with these MAbs declined considerably under denatured conditions, whereas the reactivities of MSP-Fu24, PfMSP-119, and PfMSP-311 with anti-penta-His antibody under native and denatured conditions were found to be similar in a parallel experiment that ascertained the equal coating of the antigens in both cases (Fig. 2). However, there was no reactivity of PfMSP-311 with any of the anti-MSP-1 MAbs under both native and reduced/denatured conditions. MSP-Fu24 was also recognized by polyclonal antibody raised against PfMSP-119 and PfMSP-311 (data not shown).
FIG. 2.
Reactivity of MSP-Fu24, PfMSp-119, and PfMSP-311 with anti-PfMSP-1 conformation-specific MAb 12.1 and MAb 5.2 by ELISA under nonreducing (Non-red) and reducing conditions. The assay for the reactivity of each recombinant protein with anti-penta-His antibody was carried out in parallel as a positive control to ascertain an equal amount of coating of the antigens under reducing and nonreducing conditions.
The recognition of MSP-Fu24 by human immune sera from a region where malaria is endemic was assessed by ELISA and compared with the reactivities of PfMSP-119 and PfMSP-311. Thirty-two out of 44 sera (∼73%) showed reactivity with MSP-Fu24. Sera that showed reactivity with both PfMSP-311 and PfMSP-119 or with either of the two antigens also showed reactivity with MSP-Fu24. Sera from control individuals showed no reactivity with any of the antigens (see Fig. S2 in the supplemental material).
Immunogenicity of MSP-Fu24 and mixture of its individual components.
The immunogenicity of MSP-Fu24, formulated with three different adjuvants (CFA/IFA, Montanide ISA 720, and alum) was evaluated by using BALB/c mice. In parallel sets of experiments, groups of BALB/c mice were immunized separately with each of the individual components (PfMSP-119 and PfMSP-311) as well as with their mixture for comparisons of immune responses. Antigen-specific antibodies were detected for each group after primary immunization, and antibody levels increased with each subsequent immunization. In all groups, antibody titers reached a peak after the second boost of immunization (data not shown).
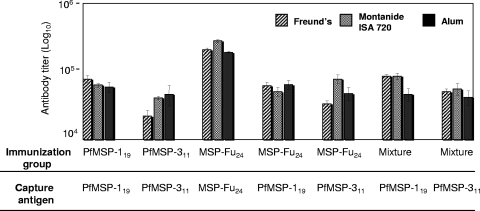
As shown in Fig. 3, immunization with MSP-Fu24 induced antibodies recognizing both PfMSP-119 and PfMSP311. Immunization with MSP-Fu24 or with the physical mixture of the two component antigens elicited antibodies recognizing both of the components in all the adjuvant groups. The antibody titers elicited for each of the component antigens in MSP-Fu24- and in mixture-immunized groups were comparable to the titer value elicited by the respective single-antigen-formulation group. MSP-Fu24 induced high antibody titers in rabbits when formulated with two human-compatible adjuvants, Montanide ISA720 or alum (end point titers of ∼3.5 × 105 and ∼16.0 × 105, respectively). The mixture of the two antigens also induced nearly similar levels of antibody titers when immunized in formulations with Montanide ISA720 or alum (end point titers of ∼19.3 × 105 and 9.4 × 105, respectively).
FIG. 3.
Immune response in groups of BALB/c mice immunized with MSP-Fu24, PfMSP119, PfMSP-311, or a mixture of PfMSP119 and PfMSP-311 formulated in different adjuvants. End point titers for anti-PfMSP119, anti-PfMSP-311, and anti-MSP-Fu24 were determined by ELISA using the respective capture antigens and mouse sera collected on day 70. The highest dilution of sera showing an OD greater than or equal to the reactivity of the preimmune sera plus 2 SDs was considered to be the end point titer. Recombinant PfMSP-119 and PfMSP-311 were used as the capture antigens to determine the immune response to the component antigens (PfMSP-119 and PfMSP-311) in the sera from group of mice immunized with MSP-Fu24 or with a physical mixture of the two components.
The IgG profiles between immunization groups for different adjuvant formulations were compared. The MSP-Fu24-Montanide and mixture-Montanide groups showed similar levels (P > 0.05) of IgG subtypes. However, the mixture-alum immunization groups showed low levels of all the isotypes compared to the MSP-Fu24-alum group (see Fig. S3 in the supplemental material).
MSP-Fu24, PfMSP-311, and PfMSP-119 induce T-cell responses in immunized mice.
T-cell responses of mice immunized with MSP-Fu24, PfMSP-119, PfMSP311, and a mixture of the individual antigens were determined. Table S1 in the supplemental material summarizes the cellular responses induced upon immunization with different formulations of MSP-Fu24, PfMSP-119, PfMSP311, and the mixture in BALB/c mice. Compared to the adjuvant control, MSP-Fu24, PfMSP-119, PfMSP311, and mixture formulations induced significant proliferation (P > 0.05). However, there was variation in the proliferative responses depending upon the adjuvant (see Table S1 in the supplemental material). Splenocytes from alum groups showed significantly lower (P > 0.05) proliferation than the corresponding Montanide groups. After stimulation with any of these antigens, MSP-Fu24, PfMSP-311, and PfMSP-119, the splenocytes of the MSP-Fu24 immunization group showed significantly higher (P > 0.05) proliferation than splenocytes from the corresponding mixture groups. Similarly, for a given adjuvant, splenocytes from the MSP-Fu24-immunized group showed significantly higher levels of proliferation when stimulated with the respective component antigens (PfMSP-119 and PfMSP-311) than the groups immunized with the individual antigen (see Table S1 in the supplemental material).
As shown in Table S2 in the supplemental material, MSP-Fu24, PfMSP-119, PfMSP311, and the mixture formulated in Montanide ISA720 elicited higher IFN-γ responses than the respective alum formulation groups (P > 0.05). For a given adjuvant, splenocytes from the MSP-Fu24 group stimulated with MSP-119 or MSP-311 showed IFN-γ secretion comparable to the secretion by splenocytes from the MSP-119 or MSP-311 group, respectively, after stimulation with the respective antigens. IFN-γ secretion was variable with physical mixture immunization groups; splenocytes from the mixture-Montanide group showed IFN-γ secretion comparable to that of the PfMSP-119-Montanide and PfMSP-311-Montanide groups. However, the mixture-alum group showed lower levels of IFN-γ compared to the PfMSP-119-alum and PfMSP-311-alum groups (see Table S2 in the supplemental material).
Anti-MSP-Fu24 antibody recognizes the native parasite protein and conformation-specific epitopes in PfMSP-119.
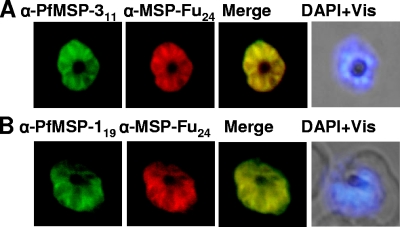
Affinity-purified anti-MSP-Fu24 antibodies from all immunization groups were assessed for their ability to recognize the native parasite protein in the blood stage of P. falciparum 3D7 parasites by immunofluorescence assay (IFA). Antibodies to MSP-Fu24 showed strong reactivity on the surface of P. falciparum merozoites. IFA end point titers varied from 1:40,000 to 1:8,0000; the highest antibody titers were observed for rabbit and mouse sera immunized with MSP-Fu24 formulated in Montanide ISA720. Fluorescence staining with anti-MSP-Fu24 antibody colocalized with the staining by anti-PfMSP-119 as well as with anti-PfMSP-311 antibodies (Fig. 4). Similar results were also obtained with purified IgG from mice and rabbits immunized with a mixture of PfMSP-119 and PfMSP-311 (data not shown).
FIG. 4.
Immunofluorescence assay with P. falciparum 3D7 parasites using anti-MSP-Fu24 antibodies. Shown are fluorescence and bright-field images of acetone-methanol-fixed P. falciparum 3D7 parasites at the schizont stage immunostained with purified anti-MSP-Fu24 (red) and anti-PfMSP-119 (green) or anti-MSP-311 (green) antibody. Parasite nuclei were stained with DAPI (blue).
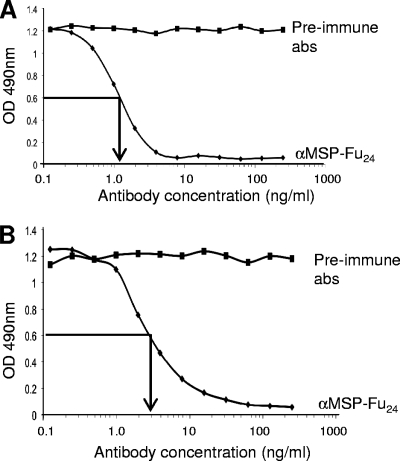
Competitive ELISA was carried out to investigate if the purified antibody from rabbit sera immunized with MSP-Fu24 contained conformation-specific and/or invasion- and processing-inhibiting antibodies directed against PfMSP-119. As shown in Fig. 5, purified anti-MSP-Fu24 antibodies inhibited the binding of conformation-specific MAb 5.2 as well as invasion- and processing-inhibitory MAb 12.10 with PfMSP-119 in a concentration-dependent manner. An approximately 50% reduction in the reactivity of MAb 5.2 was observed by competitive ELISA with anti-MSP-Fu24 antibodies at a concentration of 0.3 μg/ml; similarly, anti-MSP-Fu24 at a concentration 0.15 μg/ml caused a ∼50% reduction in the reactivity of MAb 12.10 in this assay. Thus, immunization with MSP-Fu24 induced conformation-specific as well as invasion/processing-inhibitory antibodies.
FIG. 5.
Inhibition of binding of conformation-dependent anti-PfMSP119 MAb 12.10 (A) and MAb 5.2 (B) with recombinant PfMSP-119 by purified anti-MSP-Fu24 antibodies. The reactivity of monoclonal antibodies with PfMSP119 preincubated with different concentrations of purified anti-MSP-Fu24 antibodies was assessed by use of a competitive ELISA. The arrow indicates the concentration of anti-MSP-Fu24 antibodies that brings about a 50% decrease in reactivity. Antibodies purified from preimmune sera were used as negative controls.
In vitro parasite growth inhibition by anti-MSP-Fu24 antibodies.
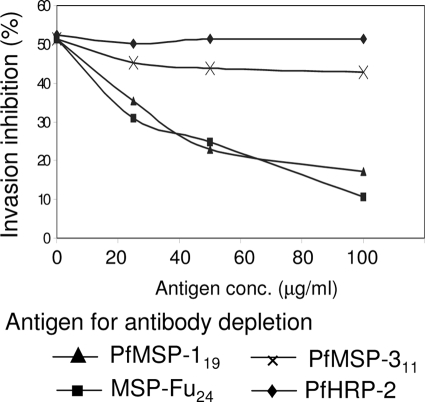
Purified rabbit IgGs were assessed for their efficacy in inhibiting in vitro parasite growth of P. falciparum strains 3D7 and FCR. Table 1 shows the percent invasion inhibition by purified antibody from rabbits immunized with different antigen-adjuvant formulations. Purified IgGs from sera of rabbits immunized with MSP-Fu24 formulations showed up to 75% and 66% parasite growth inhibitions of the 3D7 and FCR parasite strains, respectively, in a dose-dependent manner. Purified antibodies from rabbits immunized with the mixture of the two antigens also showed comparable invasion inhibition levels for both the strains. To ascertain that specific antibodies mediated this growth inhibition, antibodies specific to MSP-Fu24, PfMSP-119, or PfMSP-311 were depleted from purified anti-MSP-Fu24 rabbit antibodies by using different concentrations of recombinant MSP-Fu24, PfMSP-119, and PfMSP-311, respectively. These antibody samples depleted of specific IgGs were then assessed for their efficacies in inhibiting P. falciparum 3D7 parasite invasion. The depletion of anti-MSP-Fu24-specific antibodies showed up to an 80% reversal in the growth inhibition rate in a concentration-dependent manner, whereas PfMSP-119-depleted antibody showed a ∼70% reversal (Fig. 6). However, PfMSP-311 showed only a ∼23% reversal in growth inhibition at the same concentration. Recombinant PfHRP-2, used as a negative control in a depletion assay, did not show any reversal of invasion inhibition.
TABLE 1.
Inhibition of P. falciparum invasion of erythrocytes by purified antibodies from sera of rabbits immunized with MSP-Fu24 or with a mixture of PfMSP-311 and PfMSP119 formulated with Montanide ISA720 or aluma
| Immunization group | Parasite strain | % Parasitemiab (SD) at concn of IgG Ab (mg/ml) ofc: |
% Invasion inhibitiond at concn of IgG Abs (mg/ml) of: |
||||
|---|---|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 0.125 | 0.25 | 0.5 | ||
| Preimmune | 3D7 | 3.68 (±0.75) | 3.62 (±0.34) | 4.36 (±0.33) | |||
| FCR | 3.78 (±0.07) | 3.95 (±0.14) | 4.19 (±0.05) | ||||
| PfMSP142 | 3D7 | 2.01 (±0.29) | 2.18 (±0.104) | 1.61 (±0.28) | 45.4 | 39.78 | 63.0 |
| FCR | 2.36 (±0.09) | 2.25 (±0.07) | 1.74 (±0.08) | 37.57 | 43.04 | 58.47 | |
| MSP-Fu24-Montanide | 3D7 | 1.9 (±0.08) | 1.77 (±0.44) | 1.37 (±0.05) | 48.36 | 51.7 | 68.6 |
| FCR | 2.07 (±0.07) | 1.98 (±0.15) | 1.52 (±0.03) | 45.24 | 49.87 | 63.80 | |
| MSP-Fu24-alum | 3D7 | 1.94 (±0.43) | 1.45 (±0.12) | 1.11 (±0.14) | 47.28 | 59.94 | 74.5 |
| FCR | 2.53 (±0.07) | 2.3 (±0.08) | 1.39 (±0.08) | 32.98 | 41.77 | 66.83 | |
| Mixture-Montanide | 3D7 | 2.38 (±0.07) | 2.37 (±0.35) | 1.97 (±0.33) | 35.32 | 34.5 | 54.81 |
| FCR | 3.22 (±0.09) | 2.7 (±0.09) | 2.01 (±0.14) | 14.81 | 31.65 | 51.95 | |
| Mixture-alum | 3D7 | 2.37 (±0.31) | 2.16 (±0.45) | 2.31 (±0.35) | 35.6 | 40.1 | 47.0 |
| FCR | 3.42 (±0.16) | 2.38 (±0.09) | 2.05 (±0.13) | 9.44 | 39.58 | 51.07 | |
Anti-PfMSP-142 rabbit antibodies were used as a control.
Parasitemia values are the averages of triplicate determinations.
Final concentration of IgG in a well.
Calculated against the respective preimmune Ab well. The proportion of parasitized red blood cells in test wells was statistically significantly high compared to those in the respective control wells (P < 0.005 by chi-square test).
FIG. 6.
Reversal of anti-MSP-Fu24 antibody-mediated inhibition of P. falciparum invasion after depletion of antigen-specific antibodies. The invasion inhibition by anti-MSP-Fu24 antibodies (250 μg/ml) was assessed after the preincubation of antibodies with different amount of recombinant MSP-Fu24, PfMSP119, or PfMSP-311 to deplete antibodies specific to the respective antigens. PfHRP-2 was also used as one of the antigens for antibody depletion in the assay as a negative control. The percent reversal of invasion inhibition was calculated based upon invasion inhibition without any antibody depletion.
Anti-MSP-Fu24 antibodies inhibit parasite growth in an ADCI assay.
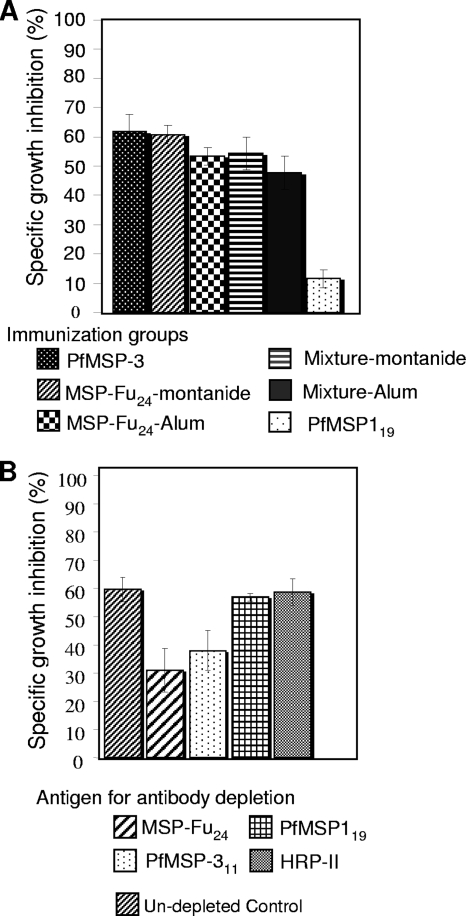
We investigated the efficacy of purified anti-MSP-Fu24 mouse antibodies in inhibiting parasite growth in cooperation with monocytes in an ADCI assay. As shown in Fig. 7A, IgGs purified from the MSP-Fu24-alum and MSP-Fu24-Montanide groups of mice significantly (P > 0.05) inhibited parasite growth (SGI of up to 63%) in cooperation with monocytes in a concentration-dependent manner. It was also observed that the ADCI effect was dependent on the IgG concentration. Purified IgG from mice immunized with a physical mixture of the two antigens also inhibited parasite growth (SGI of up to 53%) in a similar manner. However, no significant differences were observed between growth inhibition by IgGs from MSP-Fu24 and mixture immunization groups for a given adjuvant (Fig. 7A). Similar levels of inhibition were observed with anti-PfMSP-3 and anti-MSP-Fu24 antibodies; however, no significant inhibition was observed using anti-PfMSP-119 antibodies. To ascertain the specificity of growth inhibition, the anti-MSP-Fu24 antibodies were depleted by using recombinant PfMSP-119 or PfMSP-311. The depleted anti-MSP-Fu24 antibodies were then used for ADCI assays. A reversal of growth inhibition was observed after the depletion of PfMSP-311-specific antibodies (Fig. 7B), indicating that the observed ADCI effect was mediated largely by the presence of antibodies specific to PfMSP-311 in a fusion chimera.
FIG. 7.
(A) Specific growth inhibition (SGI) index of antibody-dependent cellular inhibition (ADCI) of parasite growth by total IgG purified from sera of mice immunized with MSP-Fu24, PfMSP119, PfMSP-311, or a mixture of PfMSP119 and PfMSP-311 formulated in Montanide ISA720 or alum. Parasites at the schizont stage were cocultured with human monocytes in the presence of purified antibodies (50 μg/ml of culture in the assay), and parasite growth was monitored after 96 h. (B) Reversal of antibody-dependent cellular inhibition of parasite growth by depletion of antigen-specific antibodies. The SGI index of ADCI of parasite growth purified from sera of mice immunized with MSP-Fu24-Montanide after incubation of antibodies with recombinant MSP-Fu24, PfMSP119, or PfMSP-311 to deplete antibodies specific to the respective antigen was determined.
DISCUSSION
It is generally believed that subunit vaccines containing more than one malaria antigen will have more of a chance of success than those based on a single antigen. The advantages of a combination vaccine is the possibility of a simultaneous attack on more than one parasite target and the countering of parasite growth by different mechanisms. A combination vaccine may also provide a synergetic response. However, such an approach would necessarily require the development and analysis of individual components of the combination vaccine. Proteins that appear on the surface of merozoites of P. falciparum are considered possible candidates for blood-stage malaria vaccines, and out of the many merozoite surface proteins identified so far, PfMSP-1 and PfMSP-3 are among the most promising vaccine candidate antigens. Interestingly, these two antigens seem to generate protective immune responses by entirely different mechanisms: while the antibody response to the C-terminal fragments of PfMSP-1 is supposed to be invasion inhibitory, the protective response of MSP-3 is believed to be in a monocyte-dependent manner through ADCI (23, 28). In the present study, we have produced a chimeric malaria protein containing the C-terminal fragment of PfMSP-119 fused downstream with a conserved, immunologically relevant region of PfMSP-3; in addition, we also produced the two individual component polypeptides separately. The main aim of the study was to compare immune responses of the fusion protein, MSP-Fu24, with those of a mixture of its individual components (PfMSP-119 and PfMSP-311) and also to assess the antiparasitic efficacies induced by the immune responses generated. The three recombinant proteins were expressed in E. coli cells, purified to homogeneity, and characterized by different standard procedures.
For a number of merozoite surface proteins that are rich in cysteine residues, the generation of parasite-inhibitory antibodies is dependent on specific protein conformations stabilized by several disulfide linkages, which makes it critical to produce such vaccine target recombinant proteins that conformationally resemble their native counterparts (5, 17, 26, 45). PfMSP-119 is highly structured, contains six disulfide linkages, and folds into two epidermal growth factor-like domains, which provide it with conformational stability (8, 26). A number of MAbs that show reactivity with PfMSP-119 only when it attains an appropriate conformation have been developed (45). Our results of immunoblotting and ELISA using different conformation-dependent MAbs showed that MSP-Fu24 contained immunologically relevant conformational epitopes of PfMSP-119, suggesting that the MSP-119 component in MSP-Fu24 has acquired a conformation similar to that of the native protein. The complete loss of reactivity of the conformational MAbs with the fusion protein under reducing conditions indicated that the tertiary structure of the protein is stabilized by disulfide linkages. Furthermore, MSP-Fu24 was recognized by the majority of sera from individuals residing in an area where malaria is endemic, indicating that it contained an epitope(s) present in its component antigens that may be the targets of the antibody response generated during natural exposure to P. falciparum. In addition, anti-MSP-Fu24 antibodies recognized both native PfMSP-1 and PfMSP-3 on the parasite surface.
We tested the immunogenicities of MSP-Fu24 and a physical mixture of its two-component antigens using three different adjuvants, alum, Montanide ISA720, and CFA/IFA, in small animals. Two of these adjuvants, alum and Montanide ISA720, were used previously in malaria vaccine trials for monkeys as well as humans (11, 14, 21, 37). We found that immunization with MSP-Fu24 induced a significant antibody response with all three adjuvant formulations. Considering that most vaccine target malaria recombinant antigens are poorly immunogenic, the high antibody response to the fusion protein in alum and Montanide ISA720 is encouraging. Antigenic competition or immunodominance of individual component antigens could be a potential hindrance in the development of combination-antigen-based vaccines. Immunization with MSP-Fu24 in different adjuvant formulations elicited an immune response to each of its components that was comparable to that induced by immunization with individual components. These results suggested a lack of antigenic competition when the two antigens were presented together as a fusion protein or as a physical mixture of the two. Such a lack of antigenic competition was observed previously by workers using other combination malaria vaccine constructs. For example, no antigenic competition was observed for studies with PfCP 2.9, a fusion chimera of PfMSP-119 and PfAMA-1 (31), or when this fusion protein was administered in combination with another recombinant antigen, PfEBA-175 (F2), as a single formulation (47). Similar observations were made when the C-terminal region of the PfCSP, including the repeats, was administered in combination with PfCP 2.9 (48). In another study, a fusion construct of P. yoelii MSP-1 and MSP-8 also induced an antibody response comparable to that of the two components (38). It appears that the immunodominant regions of the malaria proteins selected in these studies and in the present one have inherently strong structural preferences, which are maintained when they are presented in fusion chimeras.
The recombinant fusion chimera stimulated T lymphocytes in an in vitro assay with various stimulation indices depending on the adjuvant used. In general, the level of splenocyte proliferation was higher in MSP-Fu24-immunized groups than in the physical mixture groups. Cytokines play an important role in determining the IgG subclass. IFN-γ is a product of Th1 cells and is generally associated with the production of IgG2a, also an indicator of cell-mediated immunity. The production of IFN-γ and IgG subtype analysis of the immune response indicated that both Th1 and Th2 subsets of T-helper cells are elicited by the fusion protein.
Although many adjuvant formulations have been shown to induce high-titer antibodies against malarial antigens, it was also shown that the antibody titers alone are not sufficient to ensure protection, even when the antigen contained protective epitopes (19); the kind of antibody response generated by synthetic immunogens is equally important. Particularly for PfMSP-119, several studies have shown that it was not the total antibody titers but the levels of specific invasion-inhibitory and processing-inhibitory antibodies that correlated with protection (9, 12, 20, 30, 45). Therefore, it was important to determine the fine specificity of anti-PfMSP-119 antibody induced by the fusion chimera that may be indicative of its antiparasitic efficacy. Of several MAbs (5.2, IE1, 12.10, 2F10, 111.4, and 12.8) raised against PfMSP-1, MAbs 5.2, 2F10, 12.10, and 12.8 have been shown to be directed against conformation-specific epitopes of PfMSP-119. In addition, MAbs 12.10 and 12.8 also inhibit the proteolytic processing of PfMSP-1 during merozoite invasion (1, 7, 13). We carried out a competition ELISA-based analysis of mouse immune sera to assess the presence of anti-MSP-Fu24 antibodies that share a conformation-specific epitope(s) with these invasion/processing-inhibitory MAbs. The degree of competition in this assay is influenced mainly by the relative affinities of serum antibodies and the MAbs, the degree of overlap of the epitopes, and the concentration of serum antibodies. Similar assays were used previously to assess the induction of PfMSP-119-specific invasion-inhibitory antibodies against immunization of mice/rabbits with PfMSP-142 formulations (36). Our results show that immunization with MSP-Fu24 elicited antibodies against the PfMSP-119 component that share epitopes with conformation-specific and invasion/processing-inhibiting MAbs; these results also suggest that MSP-Fu24 can induce invasion-inhibitory/processing antibodies when used as an immunogen with human-compatible adjuvants.
In the absence of any direct correlates of protection against malaria, in vitro assays such as invasion inhibition and ADCI assays are used to assess the antiparasitic efficacy of the immune response generated to an immunogen (11, 36, 39, 40, 46). We found that purified Abs from sera of rabbits immunized with MSP-Fu24 strongly inhibited the parasite invasion of not only homologous P. falciparum strain 3D7 but also strain FCR. Furthermore, the depletion of MSP-Fu24- and PfMSP-119-specific antibodies from total anti-MSP-Fu24 antibodies decreased this inhibition, indicating the specificity of the invasion inhibition assay. On the other hand, the depletion of PfMSP-3-specific antibodies did not cause any significant reduction in invasion inhibition, suggesting that the antibody response directed against the MSP-119 component in MSP-Fu24 was mainly responsible for the invasion inhibition.
It was previously shown that some merozoite surface proteins, such as PfMSP-3, PfMSP-6, and PfGLURP, provide ADCI-mediated protection against the parasite compared to the direct invasion inhibition by PfMSP-1 (28, 29, 40, 42). For ADCI of malaria parasites, the antibodies against these proteins act in cooperation with host monocytes. These antibodies form a complex with the respective merozoite proteins and then interact with FcγRIIA on the monocytes, which induces the release of killing factors such as tumor necrosis factor alpha (TNF-α) (3). The human FcγIIa receptor is the primary trigger molecule for ADCI and exists in two alloforms, FcγRIIA-Arg/Arg131 and FcγRIIA-His/His131 (4, 32). Mouse IgG1 and IgG2b isotype antibodies bind well with the more prevalent FcγIIa receptor FcγRIIA-Arg/Arg131 (33). Our results showed that anti-MSP-Fu24 antibodies from the alum and Montanide ISA720 immunization groups significantly inhibited parasite growth in a dose-dependent manner in the ADCI assay. The ADCI-mediated parasite growth inhibition by anti-MSP-Fu24 antibodies was comparable to that by the antibodies induced by a mixture of the two component antigens. Furthermore, the depletion of anti-PfMSP-311 antibodies decreased the level of parasite growth inhibition, suggesting that anti-MSP-Fu24 antibodies directed against PfMSP-311 contribute to the protective immune response against the parasite through ADCI-related mechanisms. Although the heterogeneous combination of mouse antibodies with human monocytes in these assays may not be entirely satisfactory or close to the natural conditions, it is not possible to generate anti-MSP-Fu24 antibodies in humans for these assays in the present study. Therefore, to assess the efficacy of antibodies induced by the recombinant antigen MSP-Fu24, to elicit ADCI, we have used mouse antibodies with human monocytes as the next-best approximation for these in vitro assays. It may be important that such a heterogeneous combination was used previously for a similar set of ADCI experiments (42).
The multivalent vaccine concept is an attractive strategy for the development of a malaria vaccine, even though each of the component antigens of such a vaccine would need to be developed separately. The use of a fusion protein consisting of the immunodominant region from different antigens may provide a feasible alternative strategy. In this study, we generated a fusion protein, MSP-Fu24, consisting of immunodominant regions of PfMSP-3 and PfMSP-1. A simple protocol was established to express MSP-Fu24 as a soluble protein in relatively high yields, which can be easily scaled up for large-scale production under cyclic GMP (cGMP) conditions. Given that it has not been easy to produce PfMSP-119 as a soluble protein with satisfactory yields, it is quite remarkable that its fusion with a specific fragment of PfMSP-3 has resulted in an easy-to-purify fusion protein with the appropriate folding of the PfMSP-119 component. The fusion protein formulated with human-compatible adjuvants is highly immunogenic in small animals, and the two-component polypeptides have maintained their functional identity in the context of the immune response to the fusion protein. Our data suggest that the fusion protein has the potential to be developed as a malaria vaccine candidate.
Supplementary Material
Acknowledgments
We thank the Department of Biotechnology, Government of India, for supporting this work through a project grant for malaria vaccine development.
We also thank human volunteers for donating the serum samples; Suruksha Sachdeva, Thilan Wickramarachchi, Deepti Gangwar, and Dipto Sinha for their help; and Rakesh Singh and Ashok Das for assistance in handling animals at the animal house.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 23 November 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burghaus, P. A., and A. A. Holder. 1994. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol. Biochem. Parasitol. 64:165-169. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho, L. J., S. G. Oliveira, M. Theisen, F. A. Alves, M. C. Andrade, G. M. Zanini, M. C. Brígido, C. Oeuvray, M. M. Póvoa, J. A. Muniz, P. Druilhe, and C. T. Daniel-Ribeiro. 2004. Immunization of Saimiri sciureus monkeys with Plasmodium falciparum merozoite surface protein-3 and glutamate-rich protein suggests that protection is related to antibody levels. Scand. J. Immunol. 59:363-372. [DOI] [PubMed] [Google Scholar]
- 7.Chappel, J. A., and A. A. Holder. 1993. Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognise the first growth factor-like domain of merozoite surface protein-1. Mol. Biochem. Parasitol. 60:303-311. [DOI] [PubMed] [Google Scholar]
- 8.Chitarra, V., I. Holm, G. A. Bentley, S. Petres, and S. Longacre. 1999. The crystal structure of C-terminal merozoite surface protein 1 at 1.8A resolution, a highly protective malaria vaccine candidate. Mol. Cell 3:457-464. [DOI] [PubMed] [Google Scholar]
- 9.Corran, P. H., R. A. O'Donnell, J. Todd, C. Uthaipibull, A. A. Holder, B. S. Crabb, and E. M. Riley. 2004. The fine specificity, but not the invasion inhibitory activity, of 19-kilodalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in The Gambia. Infect. Immun. 72:6185-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 11.Druilhe, P., F. Spertini, D. Soesoe, G. Corradin, P. Mejia, S. Singh, R. Audran, A. Bouzidi, C. Oeuvray, and C. Roussilhon. 2005. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med. 2:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan, A. F., M. J. Blackman, and D. C. Kaslow. 2000. Vaccine efficacy of recombinant Plasmodium falciparum merozoite surface protein 1 in malaria-naive, -exposed, and/or -rechallenged Aotus vociferans monkeys. Infect. Immun. 68:1418-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guevara-Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermsen, C. C., D. F. Verhage, D. S. Telgt, K. Teelen, J. T. Bousema, M. Roestenberg, A. Bolad, K. Berzins, G. Corradin, O. Leroy, M. Theisen, and R. W. Sauerwein. 2007. Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine 25:2930-2940. [DOI] [PubMed] [Google Scholar]
- 15.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP-1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 16.Hisaeda, H., A. Saul, J. J. Reece, M. C. Kennedy, C. A. Long, L. H. Miller, and A. W. Stowers. 2002. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J. Infect. Dis. 185:657-664. [DOI] [PubMed] [Google Scholar]
- 17.Hodder, A. N., P. E. Crewther, M. L. Matthew, G. E. Reid, R. L. Moritz, R. J. Simpson, and R. F. Anders. 1996. The disulfide bond structure of Plasmodium apical membrane antigen-1. J. Biol. Chem. 271:29446-29452. [DOI] [PubMed] [Google Scholar]
- 18.Holder, A. A., and E. M. Riley. 1996. Human immune response to MSP-1. Parasitol. Today 12:173-174. [DOI] [PubMed] [Google Scholar]
- 19.Hunter, R. L., and A. A. Lal. 1994. Copolymer adjuvants in malaria vaccine development. Am. J. Trop. Med. Hyg. 50(Suppl. 4):52-58. [DOI] [PubMed] [Google Scholar]
- 20.John, C. C., R. A. O'Donnell, P. O. Sumba, A. M. Moormann, T. F. de Koning-Ward, C. L. King, J. W. Kazura, and B. S. Crabb. 2004. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-119) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J. Immunol. 173:666-672. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Guevara-Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stage in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 23.Mahanty, S., A. Saul, and L. H. Miller. 2003. Progress in the development of recombinant and synthetic blood-stage malaria vaccines. J. Exp. Biol. 206:3781-3788. [DOI] [PubMed] [Google Scholar]
- 24.McColl, D. J., and R. F. Anders. 1997. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3). Mol. Biochem. Parasitol. 90:21-31. [DOI] [PubMed] [Google Scholar]
- 25.McColl, D. J., A. Silva, M. Foley, J. F. Kun, J. M. Favaloro, J. K. Thompson, V. M. Marshall, R. L. Coppel, D. J. Kemp, and R. F. Anders. 1994. Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites. Mol. Biochem. Parasitol. 68:53-67. [DOI] [PubMed] [Google Scholar]
- 26.Morgan, W. D., B. Birdsall, T. A. Frenkiel, M. G. Gradwell, P. A. Burghaus, S. E. Syed, C. Uthaipibull, A. A. Holder, and J. Feeney. 1999. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J. Mol. Biol. 289:113-122. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 29.Oeuvray, C., M. Theisen, C. Rogier, J. F. Trape, S. Jepsen, and P. Druilhe. 2000. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect. Immun. 68:2617-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okech, B. A., P. H. Corran, J. Todd, A. Joynson-Hicks, C. Uthaipibull, T. G. Egwang, A. A. Holder, and E. M. Riley. 2004. Fine specificity of serum antibodies to Plasmodium falciparum merozoite surface protein, PfMSP-1(19), predicts protection from malaria infection and high-density parasitemia. Infect. Immun. 72:1557-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan, W., D. Huang, Q. Zhang, L. Qu, D. Zhang, X. Zhang, X. Xue, and F. Qian. 2004. Fusion of two malaria vaccine candidate antigens enhances product yield, immunogenicity, and antibody-mediated inhibition of parasite growth in vitro. J. Immunol. 172:6167-6174. [DOI] [PubMed] [Google Scholar]
- 32.Parren, P. W., P. A. Warmerdam, L. C. Boeije, J. Arts, N. A. Westerdaal, A. Vlug, P. J. Capel, L. A. Aarden, and J. G. van de Winkel. 1992. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J. Clin. Invest. 90:1537-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pleass, R. J., and J. M. Woof. 2001. Fc receptors and immunity to parasites. Trends Parasitol. 17:545-551. [DOI] [PubMed] [Google Scholar]
- 34.Richie, T. L., and A. Saul. 2002. Progress and challenges for malaria vaccines. Nature 415:694-701. [DOI] [PubMed] [Google Scholar]
- 35.Rotman, H. L., T. M. Daly, and C. A. Long. 1999. Plasmodium: immunization with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp. Parasitol. 91:78-85. [DOI] [PubMed] [Google Scholar]
- 36.Sachdeva, S., A. Mohmmed, P. V. Dasaradhi, B. S. Crabb, A. Katyal, P. Malhotra, and V. S. Chauhan. 2006. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-1(42) using human compatible adjuvants. Vaccine 24:2007-2016. [DOI] [PubMed] [Google Scholar]
- 37.Saul, A., G. Lawrence, A. Smillie, C. M. Rzepczyk, C. Reed, D. Taylor, K. Anderson, A. Stowers, R. Kemp, A. Allworth, R. F. Anders, G. V. Brown, D. Pye, P. Schoofs, D. O. Irving, S. L. Dyer, G. C. Woodrow, W. R. Briggs, R. Reber, and D. Stürchler. 1999. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine 17:3145-3159. [DOI] [PubMed] [Google Scholar]
- 38.Shi, Q., M. M. Lynch, M. Romero, and J. M. Burns, Jr. 2007. Enhanced protection against malaria by a chimeric merozoite surface protein vaccine. Infect. Immun. 75:1349-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh, S., K. Miura, H. Zhou, O. Muratova, B. Keegan, A. Miles, L. B. Martin, A. J. Saul, L. H. Miller, and C. A. Long. 2006. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect. Immun. 74:4573-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh, S., S. Soe, J. P. Mejia, C. Roussilhon, M. Theisen, G. Corradin, and P. Druilhe. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190:1010-1018. [DOI] [PubMed] [Google Scholar]
- 41.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theisen, M., S. Soe, C. Oeuvray, A. W. Thomas, J. Vuust, S. Danielsen, S. Jepsen, and P. Druilhe. 1998. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect. Immun. 66:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theisen, M., S. Soe, K. Brunstedt, F. Follmann, L. Bredmose, H. Israelsen, S. M. Madsen, and P. Druilhe. 2004. A Plasmodium falciparum GLURP-MSP3 chimeric protein; expression in Lactococcus lactis, immunogenicity and induction of biologically active antibodies. Vaccine 22:1188-1198. [DOI] [PubMed] [Google Scholar]
- 44.Trager, W., and J. B. Jensen. 1976. Human malaria parasite in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 45.Uthaipibull, C., B. Aufiero, S. E. Syed, B. Hansen, J. A. Guevara-Patiño, E. Angov, I. T. Ling, K. Fegeding, W. D. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. A. Lyon, and A. A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381-1394. [DOI] [PubMed] [Google Scholar]
- 46.Wickramarachchi, T., Y. S. Devi, A. Mohmmed, and V. S. Chauhan. 2008. Identification and characterization of a novel Plasmodium falciparum merozoite apical protein involved in erythrocyte binding and invasion. PLoS One 3:e1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, D., and W. Pan. 2005. Evaluation of three Pichia pastoris-expressed Plasmodium falciparum merozoite proteins as a combination vaccine against infection with blood-stage parasites. Infect. Immun. 73:6530-6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Q., X. Xue, L. Qu, and W. Pan. 2007. Construction and evaluation of a multistage combination vaccine against malaria. Vaccine 25:2112-2119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.