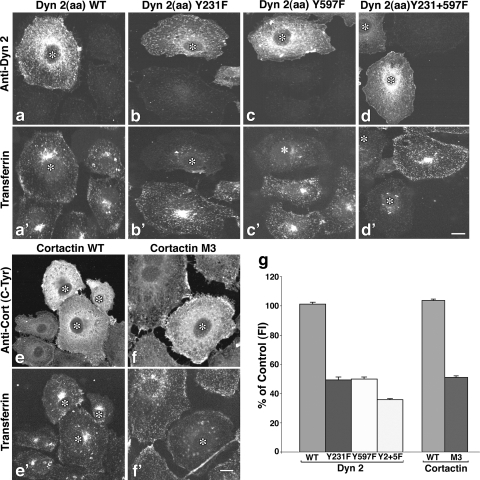
FIG. 6.
Tyrosine phosphorylation-defective mutants of Dyn2 and Cort inhibit Tf uptake. (a to f′) Clone 9 cells expressing WT Dyn2 (a) or Cort (e) or mutants in which tyrosine residues previously shown to be sites of c-Src phosphorylation were altered (Dyn2 Y231F [b], Y597F [c], Y231+597F [d], and Cort Y384,429,445F [f] mutants) were incubated with Alexa-594-labeled Tf (a′ to f′) at 37°C for 20 min and analyzed by fluorescence microscopy. Transfected cells (*) were revealed by staining with antibodies against Dyn2 (a to d) or Cort (e and f). (g) As indicated by the images, quantitation of internalized Tf based on fluorescence intensity measurements taken from 3 independent experiments showed that inhibition of either Dyn2 or Cort tyrosine phosphorylation reduced Tf uptake by 50 to 70%. Results represent the average ± SE of >100 cells measured in each of three independent experiments. Bar = 10 μm.