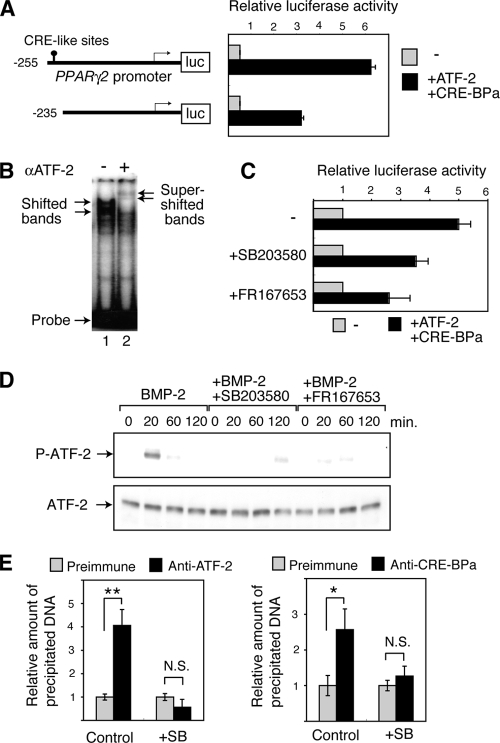
FIG. 5.
The BMP-p38-ATF-2 signaling pathway regulates adipocyte differentiation via PPARγ2 transcription. (A) Activation of the PPARγ2 promoter by ATF-2/CRE-BPa. WT MEFs were transfected with a luciferase reporter containing different fragments of the PPARγ2 promoter (shown at left) and a vector to express Smad1, Smad4, C/EBPα, ATF-2, or CRE-BPa. The relative luciferase activity is shown (mean ± SD; n = 3). (B) Gel mobility shift assays. Nuclear extracts of 293T cells that were transfected with pact-ATF-2 were incubated with a DNA probe containing the CRE-like sequence (nucleotides −255 to −235) from the PPARγ2 promoter and separated by gel electrophoresis, followed by autoradiography. In lane 2, anti-ATF-2 antibodies were added (+). (C) p38 inhibitors attenuate the ATF-2-dependent activation of the PPARγ2 promoter. MEFs were transfected with the PPARγ2 promoter-luciferase reporter and the ATF-2/CRE-BPa expression vectors or the control empty vectors, as described above. The relative luciferase activity is shown (mean ± SD; n = 3). (D) BMP-2 induces p38-dependent phosphorylation of ATF-2. 3T3-L1 cells were treated with BMP-2 at various time points. Phosphorylated ATF-2 (P-ATF-2) and total ATF-2 were detected by Western blot analysis using specific antibodies. 3T3-L1 cells were treated with BMP-2 and p38 inhibitor SB203580 or FR167653. (E) ChIP assays. Soluble chromatin was prepared from SB203580 (+SB) treated 3T3-L1 cells or untreated cells (Control) and immunoprecipitated with anti-ATF-2, anti-CRE-BPa or preimmune serum. The final DNA extraction was amplified by real-time PCR using primers that cover the PPARγ2 promoter region containing the CRE-like site. The relative densities of the bands are indicated, and each bar represents the mean ± SD (n = 3). **, P < 0.01; *, P < 0.05. N.S., no significant difference.