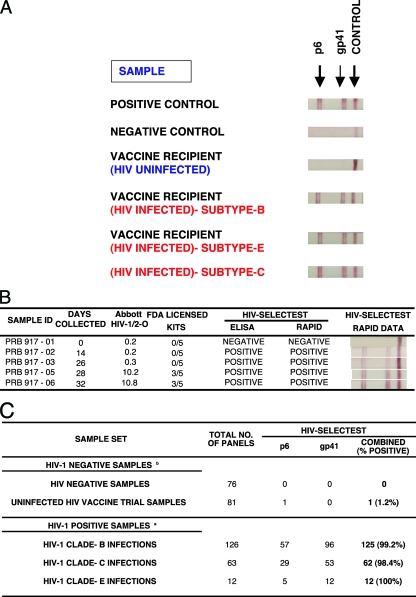
FIG. 2.
HIV-Selectest rapid test for detection of HIV-1 infections. (A) One positive control protein (goat anti-human IgG; Jackson Immunoresearch) and two sets of peptides from HIV-1 gp41 and p6 were striped onto 5-μm nitrocellulose membranes (Whatman) at 1 mg/ml per peptide. One-hundred-fold-diluted serum/plasma samples were allowed to migrate gradually through the membrane and react to HIV peptides, followed by detection with protein A-coated gold conjugate. Uninfected vaccine recipients were obtained from VRC-009 and VRC-010 (DNA/HIV prime, adenovirus type 5 [Ad5]/HIV boost). HIV-infected vaccine recipients were obtained from the VaxGen phase III trials in the United States (clade B) and Thailand (clade E). Clade C samples were obtained from the CAPRISA cohort. (B) Performance of the HIV-Selectest (EIA and rapid test) using the HIV-1 seroconversion panel (PRB 917; SeraCare BioServices, Inc.). Information on days of collection and results obtained with the Abbott HIV-1/2-O EIA and 5 FDA-licensed kits (including Abbott HIV-1, Abbott HIV-1/2, GenSys HIV-1, GenSys HIV-1/2, and OraTek HIV) were provided by SeraCare BioServices. (C) Summary of HIV-Selectest rapid test results with uninfected HIV samples (including HIV vaccine trial samples) and samples obtained from acutely HIV-infected individuals. HIV-1 clade B samples were obtained from SeraCare BioServices and NHLBI, clade C samples were obtained from the CAPRISA cohort, and clade E samples were obtained from the VaxGen vaccine trial VAX-003. HIV-negative samples were obtained from unexposed blood donors and SeraCare BioServices. The HIV vaccine trial samples were obtained from uninfected vaccine recipients from the HIV vaccine trials VRC-008, VRC-009, and VRC-010.