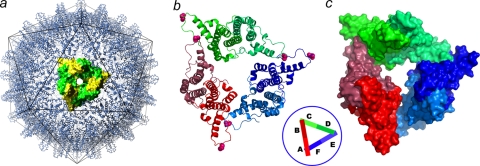
FIG. 1.
The crystallographic asymmetric unit is a trimer of dimers arranged as a broken triangle or one turn of a helix. (a) Cp149-Y132A dimers. Icosahedral HBV capsids can be considered to be arrays of triangular trimers of dimers. A threefold symmetric trimer is highlighted using yellow and green surface shading. Such triangles are the fundamental quaternary structure in HBV capsids; a trimer of dimers has been implicated in nucleating capsid assembly (58). (b and c) Asymmetric unit of Cp149-Y132A as a ribbon diagram (b) and a surface-shaded diagram (c). Dimers are labeled AB, CD, and EF (inset). The mutated residue, Y132A, is identified by the pink spheres at the ends of each dimer. The B-C and D-E interdimer contacts resemble those found in capsid. The F subunit passes under the A subunit and does not make an equivalent contact. The broken triangle of the Cp149-Y132A asymmetric unit suggests that interprotein contacts between mutant dimers are not strong enough to force a planar quaternary structure, likely the basis for their inability to nucleate capsid assembly.