FIG. 4.
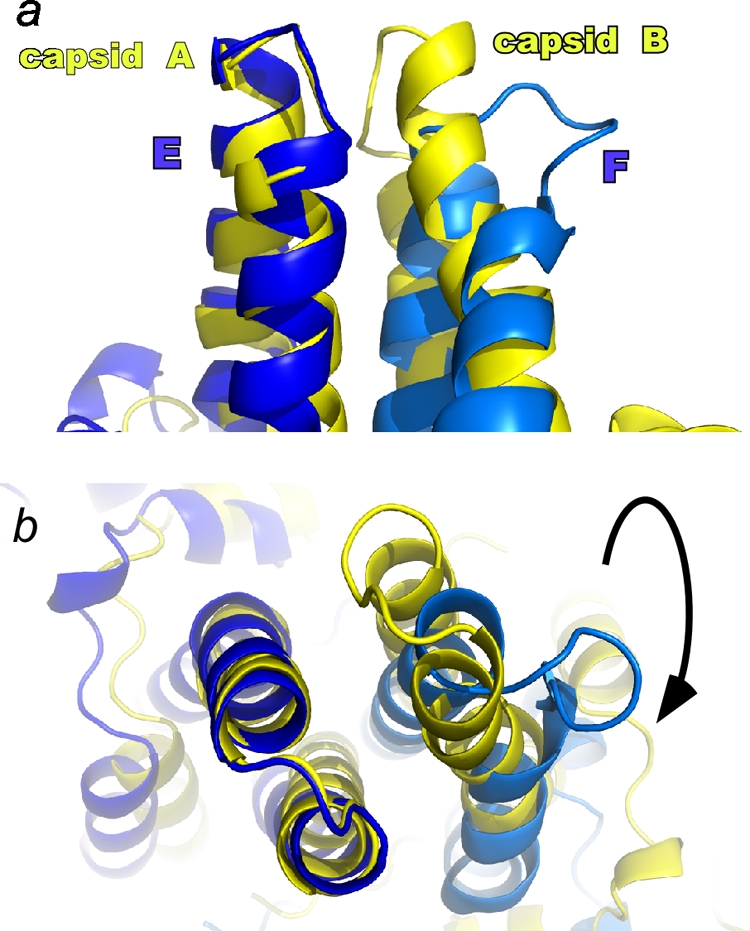
The major Cp epitopes, the spike tips at the dimer interfaces, are radically different in free and capsid forms. In many capsid-antibody complexes, the epitope includes both half dimers (24). The structural difference is made clear in this overlay of Y132A mutant and capsid dimers (colored as in Fig. 2), where alignment is based on α carbons of the structurally similar components of the spike tips (e.g., residues 70 to 85 of the Cp149-Y132A E subunit and the corresponding residues of a capsid A subunit). In the side view (a) and top view (b), the displacement of the α carbons of the F subunit spike tip is as much as 10 Å.
