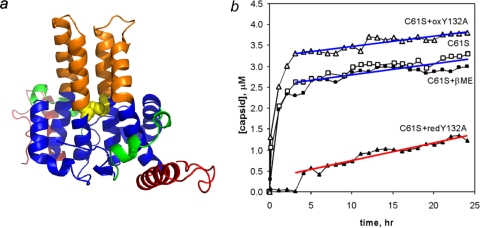
FIG. 5.
Chassis domain allostery is affected by the presence or absence of an intradimer disulfide. (a) Cysteine 61 is a conserved residue at the dimer interface in the chassis domain (domains are colored as in Fig. 3). It readily oxidizes to a C61-C61 disulfide, which may constrain chassis dynamics and conformation. (b) The presence or absence of the chassis domain disulfide in the Y132A mutant affects coassembly with Cp149-C61S at 23°C. The amounts of capsid and dimer were evaluated by size exclusion chromatography at a specified time after assembly was induced. The capsid assembly rate correlates directly to the nucleation rate (19). The assembly-competent C61S mutant was used for these comparisons, as it assembles the same way in oxidizing and reducing environments (indicated by open and closed squares, respectively). Oxidized 20 μM Cp149-Y132A (oxY132A [open triangles]) and reduced 20 μM Cp149-Y132A (redY132A [closed triangles]) were coassembled with 5 μM Cp149-C61S (C61S). While partially oxidized Cp149-Y132A was able to participate in normal assembly, reduced Cp149-Y132A poisons the reaction, slowing the nucleation rate and decreasing the extent of assembly.