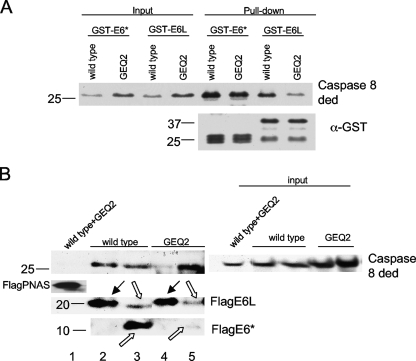
FIG. 9.
E6* and E6large bind to different regions on the procaspase 8 DED. (A) The GEQ2 set of mutations impairs binding to E6large but not to E6*, as assessed by a pulldown assay. The His-caspase 8 wt and GEQ2 mutant proteins were overexpressed in U2OS cells, and lysates were then prepared and incubated with GST-E6large or GST-E6* bound to glutathione beads. The detection of the bound caspase 8 DED wt and GEQ2 mutants was performed by immunoblotting using antibodies directed against caspase 8. The membrane was then stripped and reblotted using antibodies directed against GST (bottom). (B) The GEQ2 set of mutations impairs binding to E6large but not to E6*, as assessed by coimmunoprecipitation. U2OS cells transiently expressing either Flag-tagged E6large or E6* were transfected with plasmids encoding the GEQ2 mutant (pTriEx-cas8 ded GEQ2) or the wild-type caspase 8 DED (pTriExcas8 ded wt). Forty-eight hours posttransfection, immunoprecipitation was performed by using anti-Flag agarose, which recognizes and binds to Flag-E6. Following SDS-PAGE, the resultant immunoblot was probed with antibodies directed against caspase 8 and E6. Flag-PNAS was included as a negative control. Black arrows indicate Flag-E6large, and uncolored arrows indicate Flag-E6*. E6* can migrate as both monomers and dimers in SDS-PAGE gels.