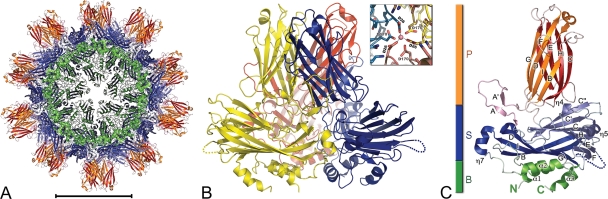
FIG. 1.
Structure of IPNV VP2. (A) The SVP is represented in a ribbon representation with base (B; green), shell (S; blue), and projection (P; rust) domains highlighted. Sixty identical copies of VP2 are organized with T=1 icosahedral symmetry. Scale bar, 100 Å. (B) The VP2 trimer, with the three protomers colored differently (blue, yellow, and red). The inset shows a Co2+ ion located at the center of the VP2 trimer and coordinated by Asp26 residues (distance, 2.2Å) from the three protomers of the trimer. Residues Asp170 bridge to consecutive Asp26 about the 3-fold axis. (C) Ribbon representation of a VP2 molecule color-coded as described in panel A. The AA′ flap is shown in pink. β-Strands are labeled according to their order in the primary sequence. N and C termini are labeled in green (B domain color). The loop connecting β-strands E and F, for which there was a break in the electron density map, is highlighted with a dotted line (also in panel B).