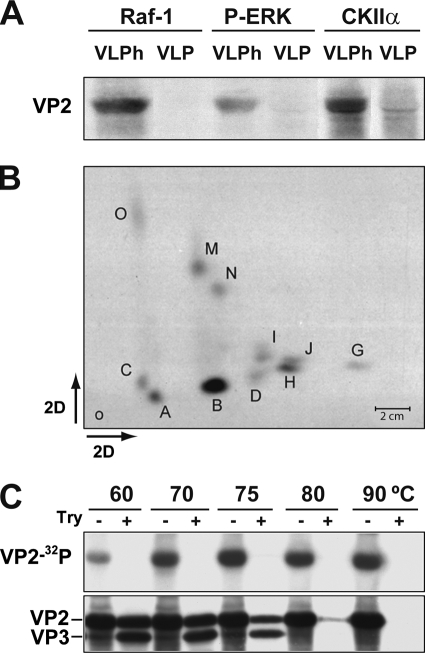
FIG. 3.
Raf-1 phosphorylation of MVM assembly intermediates in vitro. (A) Phosphorylation of VP2 subunits of native (VLP) or heated-denatured (VLPh) VLPs by immunoprecipitated kinases. Autoradiography after SDS-PAGE is shown. (B) Two-dimensional phosphopeptide map of the VP2 protein phosphorylated in vitro by pulldown Raf-1. Phosphopeptides are named according to a previous nomenclature (42). (C) Raf-1 phosphorylation of MVM capsid disassembly intermediates. Purified wtVLP particles heated at the indicated temperatures were subjected to Raf-1 phosphorylation (see Materials and Methods) and, wherever indicated (+), to subsequent trypsin digestion at 37°C as described previously (20, 42). Samples were resolved by SDS-8% PAGE and electroblotted, and filters were exposed to autoradiography (upper) and subsequently developed with the anti-VPs antibody (lower).