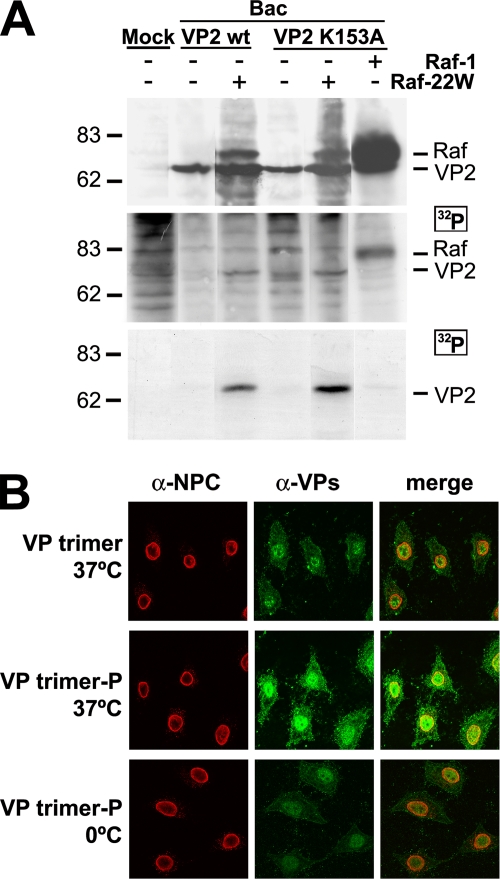
FIG. 5.
Raf-1 phosphorylation suffices VP2 trimer for nuclear import. (A) VP2 phosphorylation by Raf-1 in insect cells. H5 cells infected with the indicated baculoviruses were labeled at 22 to 28 hpi with [32P]orthophosphate. The upper section blot shows VP2 and Raf-1 expression in the [32P]orthophosphate-labeled cultures. For the middle panel, autoradiography of the same filter was performed. The lower panel shows phosphorylation of VP2wt and VP2-K153A proteins in Raf-22W-expressing insect cells demonstrated by immunoprecipitation with the α-VPs antibody. Approximately 105 labeled cells were subjected to each analysis. (B) Confocal laser scanning microscopy of the nuclear transport of phosphorylated VP2 trimer in mammalian cells. Gradient-purified VP2 trimers from insect cells infected by the Bac-VP2wt (VP trimer) or by the Bac-VP2wt and the Bac-Raf1-22W baculoviruses (VP trimer-P) were subjected to transport analysis in permeabilized HeLa cells. The lower panels show the results of a control experiment with permeabilized cells kept on ice showing no significant nuclear localization caused by diffusion.