Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) viral glycoproteins play important roles in the infectious life cycle and have been implicated in KSHV-associated endothelial cell transformation, angiogenesis, and KS-induced malignancies. KSHV-associated primary effusion lymphomas (PELs) secrete high levels of vascular endothelial growth factor (VEGF) and viral interleukin-6 (vIL-6) in vitro and VEGF, vIL-6, and basic-fibroblast growth factor (b-FGF) in mouse xenografts. KSHV-encoded glycoproteins B (gB) and K8.1 stimulate VEGF secretion, most likely mediated by direct or indirect binding to cell surface receptors, including the gB-specific αVβ3 and α3β1 integrins. In this study, the short interfering RNA (siRNA)-mediated inhibition of either gB or K8.1 transcription by anti-gB or -K8.1 siRNAs caused a substantial reduction in virion egress and a decrease in both vIL-6 and VEGF production. Similarly, the treatment of BCBL-1 cells with anti-gB or anti-K8.1 antibodies caused a substantial reduction in vIL-6 and VEGF production. Codon-optimized versions of either wild-type gB, mutant gB having the RGD amino acid motif changed to RAA, or K8.1 efficiently rescued virion egress and VEGF and vIL-6 production. These results suggest that the binding of gB via its RGD motif to integrin receptors was not responsible for the observed gB-associated regulation of VEGF and vIL-6 transcription. Conditioned medium collected from BCBL-1 cells transfected with anti-gB and anti-K8.1 siRNAs or treated with anti-gB and anti-K8.1 antibodies exhibited a significantly reduced ability to induce the formation of the capillary network of endothelial cells compared to the ability of medium from mock-infected BCBl-1 cells. Furthermore, medium obtained from BCBL-1 cells expressing smaller amounts of gB and K8.1 produced a substantial reduction in endothelial cell migration in a vertical migration assay compared to that of control medium containing wild-type levels of gB and K8.1. These results suggest a functional linkage between gB/K8.1 synthesis and VEGF/vIL-6 transcriptional regulation via paracrine and/or autocrine signaling pathways.
Kaposi's sarcoma-associated herpesvirus (KSHV), also referred to as human herpesvirus 8 (HHV-8), is a member of the gamma-2-herpesvirus family (genus Rhadinovirus) (36, 51). KSHV is etiologically associated with Kaposi's sarcoma, primary effusion or body cavity-based lymphoma, and multicentric Castleman's disease (4, 18, 54). Typically, all herpesviruses initiate infection by the binding of several viral glycoproteins embedded within the viral envelope to specific receptors on cell surfaces. Viral glycoproteins also mediate the fusion of the viral envelope with either cellular or endosomal membranes and function in the final stages of virion morphogenesis and egress (23, 34, 50).
KSHV codes for a number of glycoproteins, some of which have significant homology to glycoproteins of other herpesviruses. These include glycoproteins gB (ORF8) (38), gH (ORF22), gM (ORF39), gL (ORF47) (36, 51), and gN (ORF53) (12, 26, 51). KSHV also encodes additional glycoproteins that do not have homologs in other herpesviruses, including gpK8.1A, gpK8.1B, K1, K14, and K15, which are expressed during lytic replication (51). Glycoprotein B (gB) is one of the most conserved herpesvirus glycoproteins. It is an essential virion component for members of the alpha- and betaherpesvirus subfamilies and functions in virion attachment and virus entry into susceptible cells (10, 14, 41). KSHV virions incorporate gB in the viral envelope, which is important for attachment to cell surfaces and entry via an RGD-dependent binding to integrins. Initially, the α3β1 integrin was implicated (3), but more recently, it was shown that αVβ3 is the integrin involved in gB RGD-mediated virus entry (19). KSHV gB is a type 1 membrane glycoprotein 845 amino acids (aa) in length (7, 44, 46). It contains a predicted signal sequence of 23 aa, an extracellular domain containing multiple glycosylation sites and multiple hydrophobic regions, of which the carboxyl-most terminal region serves to anchor gB in membranes (7, 44-46). gB has been shown to be involved in the egress of herpesviruses, including pseudorabies virus (PRV; alphaherpesvirus) and Epstein-Barr virus (gamma-1-herpesvirus) (9, 25, 28, 40, 41). KSHV gB also was shown to be essential for virion egress in 293 cells (27). Recently, we demonstrated that KSHV gB is important for virion egress in BCBL-1 cells, while the carboxyl terminus of gB is not (33). In contrast to these examples, herpes simplex virus type 1 (HSV-1) gB is not required for virion egress (11).
Glycoprotein K8.1A has 228 amino acids (aa) and contains a signal sequence, a transmembrane domain, and four N-glycosylation sites. A smaller glycoprotein, K8.1B, is formed by the differential splicing of the K8.1 transcript, resulting in a glycoprotein of 167 aa having three N-glycosylation sites. However, K8.1A is found predominantly in infected cells and within virion envelopes (12, 67, 68). K8.1A binds to heparin sulfate moieties on cell surfaces, facilitating virion binding to cells (62). Soluble versions of K8.1A were shown to activate the gamma/beta interferon (IFN-α/β) signaling pathway, inducing an antiviral state (43).
The KSHV-induced upregulation of different angiogenic factors is thought to be involved in KS development and progression. Specifically, a number of recent studies have shown that the KSHV infection of endothelial cells induces angiogenesis and invasive phenotypes by inducing angiogenic and inflammatory cytokines, such as Ang-2, MMP1, MMP9, and IL-6 (47, 48, 64, 65). A number of KSHV-encoded proteins, including K1, vGPCR, and vIL-6, have been reported to induce the major angiogenic factor VEGF-A, suggesting its involvement in KSHV pathogenesis (5, 8, 56, 63). Soluble versions of viral glycoprotein K8.1A alone or gB and K8.1A added together to cultured human microvascular endothelial cells (HMVEC-d) upregulated VEGF-A synthesis. However, treatment with soluble gB alone did not induce significant levels of VEGF-A. These results suggested that the binding of KSHV via K8.1 and gB on cell surfaces is sufficient to induce signal transduction pathways that lead to the upregulation of VEGF-A synthesis (55).
Previous experiments from our laboratory have indicated that glycoprotein K8.1A was dispensable for viral infectivity in 293T cells. This conclusion was based on the generation of a KSHV mutant virus that carried a deletion of the K8.1 gene constructed on the KSHV genome cloned into a bacterial artificial chromosome (bac) (30). In this study, we show that the inhibition of K8.1 synthesis in BCBL-1 cells by anti-K8.1 short interfering RNAs (siRNAs) inhibited virion egress in a manner similar to that produced by the inhibition of gB synthesis (57). Importantly, we show for the first time that the inhibition of either gB or K8.1 synthesis in BCBL-1 cells causes significant inhibition of both VEGF and vIL-6 production. The fact that the treatment of BCBL-1 cells with anti-gB and anti-K8.1 antibodies causes a similar inhibition of VEGF and vIL-6 production suggests that gB and K8.1 function via paracrine/autocrine signaling pathways to regulate VEGF and vIL-6 production.
MATERIALS AND METHODS
Cell lines and media.
BCBL-1 cells harboring rKSHV.152 (61) and expressing green fluorescent protein (a gift from Jeff Vieira, University of Washington) were cultured in RPMI 1640 (Hyclone) medium with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone) and 2 mM l-glutamine, 1% penicillin-streptomycin (pen/strep) (Gibco), and 250 mg of G418/ml (Gibco). 293T cells (ATCC) were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS and 1% pen/strep. The KSHV lytic cycle was induced in BCBL-1 cells by the addition of 25 ng/ml of 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma, St. Louis, MO) for 12 to 48 h. Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza Biotech and maintained per the supplier's instructions.
Antibodies.
Rabbit polyclonal antibodies generated in our laboratory against KSHV gB peptides were used to detect gB protein in Western blot analysis (7). Mouse monoclonal antibodies against Hu-VEGF (ABCAM) or Hu-GAPDH (R&D systems), and rabbit antibody against K8.1 (Santa Cruz Biotechnology, Inc.) or gB (produced in this laboratory), were used to detect the respective proteins from conditioned medium or from cell lysates.
Vector construction.
The short hairpin RNAs (shRNAs) against gB (57) and K8.1A were cloned in p3xFLAG vector. The two K8.1 shRNA oligonucleotides (si-5 and si-10) were designed with flanking restriction sites HindIII and NotI for si-5 (top strand, AGCTTCTGTTCCTACGAGAATATGTTCAAGAGACATATTCTCGTAGGAACAGTTGC; bottom strand, GGCCGCAACTGTTCCTACGAGAATATGTCTCTTGAACATATTCTCGTAGGAACAGA) and XbaI and BamHI for si-10 (top strand, CTAGAGATGCAATAGATGAATCGGTTCAAGAGACCGATTCATCTATTGCATCTTG; bottom strand, GATCCAAGATGCAATAGATGAATCGGTCTCTTGAACCGATTCATCTATTGCATCT). The short hairpin oligonucleotides were cloned in p3xFLAG vector as described earlier (57). BAC 36 carrying the KSHV genome was used to amplify the wild-type K8.1A gene using the primers K8.1-FP1 and K8.1-RP3. The site-directed mutagenesis of the wild-type K8.1A gene at the siRNA target regions of si-5 (bp 163 to 183) and si-10 (bp 299 to 319) was carried out using the six primers of K8.1 FP1-RP3 described in Table 1. The codon-altered K8.1 was cloned in p3xFlag vector either individually or in combination with si-5 or si-10. In the case of glycoprotein B, site-directed mutagenesis at the RGD motif (amino acids 27 to 29) of codon-optimized glycoprotein B (ps18+gBco) (57) was carried out using the gBr-F and gBr-R primers (Table 1) to generate pgBR.
TABLE 1.
Primers used in this study
| Name of primer | Oligonucleotide sequencea | Expected DNA fragment size (bp) |
|---|---|---|
| gBUNI (gB universal; forward) | TCCAGACTACCCACGAGGAC | 240 |
| gBUNI (gB universal; reverse) | GTCAGGTTAATCGCGGACAT | |
| GAPDH (forward) | GATTCCACCCATGGCAAATT | 80 |
| GAPDH (reverse) | AAGATGGTGATGGGATTTCCATT | |
| ORF59 (forward) | TCAGCTTCAGGAATACGTCCG | |
| ORF59 (reverse) | GGCTATGCCAGCGTCGAGTA | |
| ORF59 (probe) | FAM-CGCGTGAGCTATTCGGTGCGAATA-TAMRA | |
| K8.1A FP1 | AACTGCAGCTACGAGAACATGACGGCCCTAGAGGCCGTGCTC | 183 |
| K8.1A RP1 | GCCGCTCTCGTCGATGGCGTCCTCTCCAGACCCAGAGGCAGAC | |
| K8.1A FP2 | ATGAGTTTCTAGAATGAGTTCCACACAGATTCGC | 137 |
| K8.1A RP2 | CATGTTCTCGTAGCTGCAGTTCATCCTGCCTAGCCAGTCCT | |
| K8.1A FP3 | AGGACGCCATCGACGAGAGCGGCTCGGGGGAGGAAGAGCGTCC | 370 |
| K8.1A RP3 | ATAGTTGGATCCGTGTTACTCTATGTAGGGTTT | |
| gBr-F | CTCACAGCAGGGCTGCCACCTTCCAGA | |
| gBr-R | TCTGGAAGGTGGCAGCCCTGCTGTGAG | |
| K8.1 (forward) | TTATCAGGACTGGCTAGGCAG | 260 |
| K8.1 (reverse) | AGGCTGATATTAAGGCATCG | |
| vIL6 (forward) | TTGTGGTCTCTCTTGCTGGT | 460 |
| vIL6 (reverse) | TGAAGCCTCCCTAATAGACC | |
| Hu VEGF (forward) | TGGACATCTTCCAGGAGTACCCTGA | 350 |
| Hu VEGF (reverse) | ACAAATGCTTTCTCCGCTCTGAGCAA |
FAM, 6-carboxyfluorescein; TAMRA, carboxytetramethylrhodamine. Italicized nucleotides represent the RAA mutated amino acid sequence.
Transient transfection of BCBL-1 cells.
The transient transfection of BCBL-1 cells was carried out in six-well plates using the Superfect (Qiagen) transfection agent according to the manufacturer's instructions and as described previously (57).
Reverse transcription-PCR (RT-PCR).
The lytic replication of KSHV was induced by resuspending transiently transfected BCBL-1 cells in serum-containing RPMI 1640 medium supplemented with 25 ng/ml TPA 24 h posttransfection. Cells were transfected with plasmids expressing either a nonspecific siRNA, an siRNA against K8.1, an siRNA against gB, siRNAs against both gB and K8.1, or medium without additives. The cells were pelleted at 1,500 rpm for 10 min. Harvested cells were resuspended in 500 μl of ice-cold PBS and split into two parts for RNA extraction (200 μl) and Western blot analysis (300 μl). Total RNA was extracted using the Nucleospin RNA II kit from Clontech Laboratories by following the manufacturer's instructions. First-strand cDNA was prepared using the high-capacity reverse transcription kit from Applied Biosystems by following the manufacturer's instructions. Equal quantities of first-strand cDNA were used for PCR using the universal gB primers, primers for K8.1, VEGF, IL-6, IL-6R, and the primers for glyceraldehyde phosphate dehydrogenase (GAPDH) (Table 1). The PCR products were resolved by electrophoresis on a 1% agarose gel, quantified by densitometry, and normalized to levels of GAPDH. GAPDH levels remained relatively constant in all experiments.
Western blot analysis.
Total soluble protein was extracted and quantified from transiently transfected 293 and BCBL-1 cells as described earlier (57). Mouse monoclonal antibodies against Hu-VEGF (ABCAM), IL-6 (R&D systems), and Hu-GAPDH were used as primary antibodies. Horseradish peroxidase (HRP)-tagged anti-rabbit/anti-mouse antibodies were used as secondary antibodies. Immobilon (Millipore) Western blotting detection reagent was used for the chemiluminescent detection of proteins.
Microtubule formation to study angiogenesis.
The microtubule formation assay was adopted from previously published work (31). Specifically, transiently transfected BCBL-1 cells were resuspended in RPMI 1640 medium containing 25 ng/ml TPA 24 h posttransfection. For the antibody treatment experiments, anti-gB or anti-K8.1 antibodies were added to the medium during the TPA treatment cycle at a concentration of 1 μg/ml. After a 24-h induction with TPA, this medium was removed and replaced with fresh RPMI medium without serum for an additional 24 h.
HUVECs (5 × 104/well) were seeded in an eight-well chamber slide pretreated with 0.1% glycerin. Total protein in the conditioned medium from BCBL-1 cells was quantified using a bicinchoninic protein assay kit (Pierce Inc.), and equal amounts of protein from each sample were layered over the HUVECs. The volume was made up with serum-free HUVEC medium. After 48 h, the cells were fixed in ice-cold methanol for 20 min and stained with hemolysin and eosin to visualize microtubule formation representing in vitro angiogenesis. The microtubules were visualized and imaged under 2.5× magnification, and five random viewing fields from each sample were counted. Angiogenesis was quantified by counting the number of branch points and the total number of branches per point, with the product indicating the degree of angiogenesis. The scoring of angiogenesis was performed in a double-blinded fashion, having two independent observers score each treatment.
Migration assay for endothelial cells (Boyden chamber assay).
A vertical migration assay described previously (32) was adopted to determine the potential of medium conditioned by BCBL1 cells (c.m.) to serve as a chemoattractant for HUVECs. Specifically, BCBL-1 cell supernatants were collected in the same way as that described for the microtubule formation assay. For this assay, 30,000 HUVEC cells (purchased from Lonza Group) were suspended in 200 μl of a 1:5 (vol/vol) mixture of HUVEC growth medium. Opti-MEM (Invitrogen Corporation) was applied to the upper chamber of a transwell insert (8.0-μm pore size; catalog no. 353097; BD Falcon). The bottom chamber was filled with 600 μl of BCBL1 c.m. standardized to uniformly contain 1.4 mg/ml total protein. In addition, a positive chemoattractant control consisting of VEGF (10.0 ng/ml; Biosource Invitrogen) in RPMI 1640 medium was included. HUVEC migration was allowed to proceed for 6 h at 37°C, after which inserts were removed and cells remaining on the upper surface of the membrane were swabbed away with a cotton applicator. Cells that migrated to the insert's bottom surface were stained with hematoxylin and eosin and counted. Results are expressed as cells per 400× objective field. Five regions from each of three inserts per condition were averaged.
TaqMan real-time PCR analysis.
Real-time PCR was carried on viral DNA from cell pellets and supernatants of BCBL-1 cells as mentioned above. The primers and probe (6-carboxytetramethylrhodamine [TAMRA]) for the real-time PCR were designed to detect ORF 59 (Table 1). Supernatants were collected 48 h postinduction, and 200 μl was used for the extraction of viral DNA. The supernatants were treated with turbo DNase I (Ambion) for 2 h at 37°C. Viral DNA was extracted using the DNeasy blood and tissue kit (Qiagen) per the manufacturer's instructions. Equal volumes of viral DNA were used for TaqMan PCR analysis. KSHV BAC 36 DNA was used to generate the standard curve.
RESULTS
The effect of glycoproteins gB and K8.1 synthesis on virion egress from BCBL-1 cells.
Previous work in this laboratory has shown that gB synthesis can be inhibited effectively by anti-gB siRNAs. Furthermore, gB synthesis can be restored upon the transient expression of codon-optimized gB genes containing analogous gene sequences not recognized by the anti-gB siRNAs (57). In this study, different plasmid vectors were utilized to regulate gB and K8.1 synthesis (Fig. 1). Plasmid ps18/gBco expressing the si18 anti-gB siRNA and a codon-optimized version of gB has been described previously (57). Site-directed mutagenesis was utilized to construct the plasmid vector ps18/gBR expressing a gBco gene specifying RGD-to-RAA amino acid changes. Similar plasmid vectors were constructed to express the anti-K8.1 siRNAs siK5 (ps5) and siK10 (ps10), either separately or together (ps5/s10). One more plasmid (ps5/K8.1co) was constructed to express the K8.1 codon-modified sequence (K8.1co) together with the siK5 siRNA.
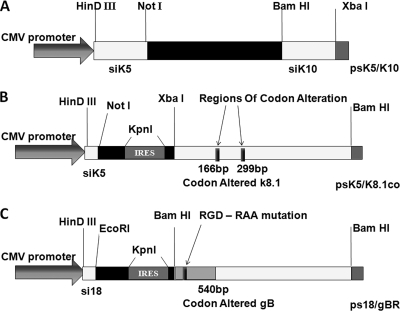
FIG. 1.
Schematic diagram of plasmid vectors used in transfection experiments. (A) p3xFLAG vector carrying anti-K8.1 siRNAs siK5 and siK10 (ps5/s10). The siK5- and siK10-encoding gene cassettes also were cloned individually at the restriction sites indicated to generate vectors ps5 and ps10 (not shown). (B) The nucleotide sequence of K8.1 was altered at the target region of siK5 (166 bp) and siK10 (299 bp) and cloned downstream of siK5 to generate the vector ps5/K8.1co. The codon-altered K8.1 and siK5 gene cassettes are separated by a 52-bp encephalomyocarditis virus internal ribosome entry site (ECMV IRES). (C) The ps18/gbco plasmid vector coexpressing anti-gB siRNA si18 and codon-optimized gB was used to generate the plasmid vector ps18/gBR, which carries the RGD-RAA mutation from amino acids 27 to 29 instead of the gB wild-type gene.
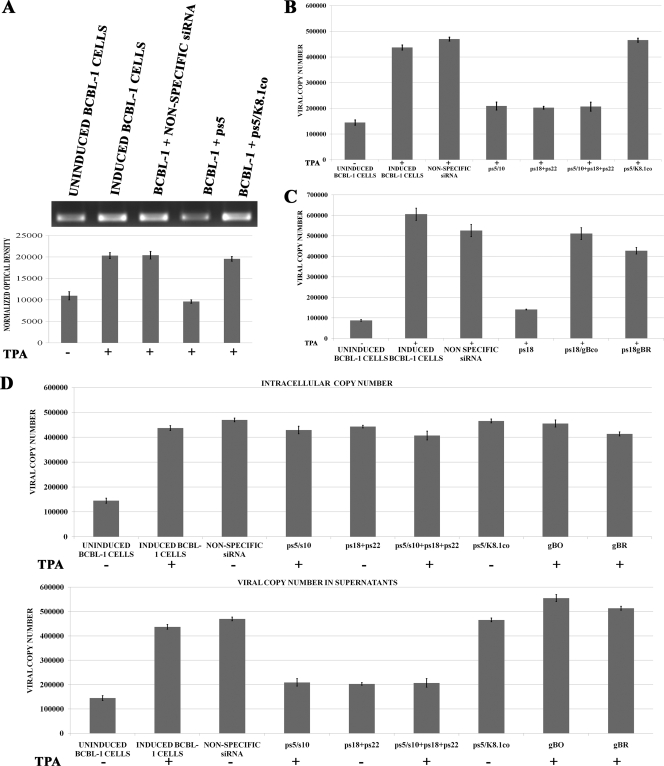
The transfection of BCBL-1 cells with plasmid ps5 reduced K8.1 mRNA levels by approximately 50%, as evidenced by the reduction in the K8.1-specific RT-PCR product. Transfection with plasmid ps5/K8.1co produced mRNA levels similar to those observed in BCBL-1 cells transfected with a plasmid encoding a nonspecific siRNA or TPA-induced, but not transfected, BCBL-1 cells (Fig. 2A). Previously, we demonstrated that the inhibition of gB synthesis caused the inhibition of virion egress from BCBL-1 cells (57). Similar experiments were performed to investigate the potential role of K8.1 in virion egress. The transfection of BCBL-1 cells with a plasmid expressing both anti-K8.1 siRNAs (ps5/s10) resulted in a substantial reduction of virion egress comparable to that produced by transfection with the anti-gB siRNAs. The simultaneous transfection of both anti-gB (ps18+ps22) and anti-K8.1 (ps5/s10) plasmid vectors also produced similar levels of egress inhibition. The transfection of BCBL-1 cells with plasmid vector ps5/K8.1co expressing the codon-altered K8.1 and the siK5 siRNA efficiently rescued virion egress (Fig. 2B). To further assure that siRNA expression did not affect viral replication, the total amount of viral DNA within BCBL-1 cells was determined and compared to the amount of viral DNA found within virion particles from their respective supernatants. The total viral DNA within BCBL-1 cells remained relatively constant irrespective of the treatment with the various plasmids expressing siRNAs and the codon-optimized gB or K8.1 (Fig. 2D).
FIG. 2.
Effect of inhibition of K8.1 and/or gB synthesis on virion egress. (A) BCBL-1 cells were transfected with plasmid vectors expressing nonspecific siRNA, ps5, or ps5/K8.1co (expressing a codon-altered K8.1 gene and siK5). Cell pellets were collected 16 h postinduction, and total RNA was extracted. First-strand cDNAs were produced and amplified by PCR using oligonucleotide primers described in Table 1, and the resultant 350-bp DNA fragment was quantified by densitometry and normalized to GAPDH. (B) BCBL-1 cells were transfected with plasmid vectors expressing nonspecific siRNA, ps5/s10, ps18+ps22, ps5/s10 + ps18+ps22, or ps5/K8.1co (expressing a codon-altered K8.1 gene and siK5). Cells were induced (+) or not induced (−) with TPA 24 h posttransfection, supernatants were collected 48 h postinduction, and viral DNA was extracted and quantified via PCR. (C) Experiments were performed as described for panel B to investigate whether altering the gB RGD to RAA had any effect on virion egress. BCBL-1 cells were transfected with plasmid vectors expressing nonspecific siRNA, ps18, ps18/gBco, and ps18/gBR and were assayed for viral DNA as described for panel B. All experiments were conducted in triplicate, and error bars represent standard errors of the means (SEM). (D) Quantitative PCR analysis results of intracellular and extracellular viral DNA. Quantitative PCR was performed using oligonucleotide primers specific for KSHV ORF59 as described previously (57) (also see Materials and Methods).
The gB RGD amino acid motif does not function in virion egress.
The amino terminus of gB contains an RGD amino acid motif, which is important for binding to its cognate receptors αVβ3 and/or α3β1 on KSHV-susceptible cells (2, 19). This gB binding induces integrin-dependent signaling pathways that regulate a number of important cellular and viral processes (60). To investigate whether the gB RGD motif was important in virion egress from BCBL-1 cells, BCBL-1 cells were transfected with plasmid ps18/gBco or ps18/gBR, both of which inhibit the endogenous wild-type gB synthesis through the action of the s18 siRNA while simultaneously encoding either the codon-optimized gB or codon-optimized gB carrying the RGD-to-RAA mutations. gBR rescued virion egress as efficiently as the gBco-expressing plasmid (Fig. 2C).
Inhibition of gB and K8.1 synthesis causes inhibition of VEGF mRNA levels.
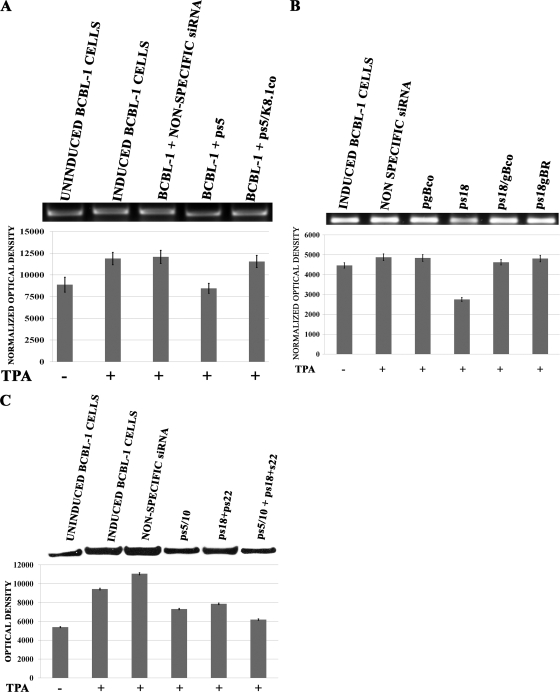
Previous studies have shown that soluble versions of either gB or K8.1 enhanced the production of VEGF (55). To investigate the potential effects of gB and K8.1 inhibition on VEGF and vIL-6 production, the relative levels of VEGF and vIL-6 mRNAs were assessed by RT-PCR (see Materials and Methods) (Fig. 3). The transfection of BCBL-1 cells with the anti-K8.1 siRNAs (ps5) caused an approximately 35% inhibition of VEGF mRNA levels. In contrast, transfection with the codon-altered K8.1 carrying plasmid ps5/K8.1co restored VEGF levels to those produced in control samples derived from either TPA-induced cells or cells transfected with a plasmid encoding a nonspecific siRNA control (Fig. 3A).
FIG. 3.
Effect of K8.1 and/or gB inhibition on VEGF production. (A) BCBL-1 cells were transfected with plasmid vectors expressing nonspecific siRNA, ps5 (K8.1 target), or ps5/K8.1co. Cells were induced with TPA (+) or left uninduced ([) starting at 24 h posttransfection. Cell pellets were collected 16 h postinduction, and total RNA was extracted. First-strand cDNAs were produced and amplified by PCR using primers described in Table 1, and the resultant 250-bp DNA fragments were quantified by densitometry and normalized to GAPDH. (B) Experiments similar to those described for panel A using plasmid vectors expressing nonspecific siRNA, ps18, ps18+gBco, and ps18+gBR. (C) Experiments similar to those described for panels A and B with plasmid vectors expressing nonspecific siRNA, ps5/s10, ps18+ps22, and ps5/s10 + ps18-22. Cells were induced with TPA (+) 24 h posttransfection or left uninduced (−), supernatants were collected 48 h postinduction, and the total amount of protein was determined. Equal amounts of protein from the supernatants were loaded on SDS-PAGE gels. Experiments were conducted in triplicate, and error bars represent SEM. The bar graphs represent the densitometric analysis results obtained using the Image J software.
gB is known to bind to αVβ3 integrin and induce integrin-dependent autocrine and paracrine signaling that regulates VEGF and vIL-6 production (55). To assess whether the gB RGD amino acid motif was involved in the observed regulation of VEGF mRNA levels, BCBL-1 cells were transfected with a plasmid expressing the anti-gB siRNA (ps18) as well as either the codon-optimized gB (ps18/gBco) or codon-optimized gB carrying the RGD-to-RAA mutations (ps18/gBR). The anti-gB siRNA si18 decreased the amount of VEGF transcripts by approximately 30% compared to that of cells transfected with a nonspecific siRNA (control). The combined use of both anti-gB and anti-K8.1 siRNAs failed to augment VEGF transcriptional inhibition beyond the level of inhibition produced by ps5 alone (not shown). The gBco and gBR plasmids rescued VEGF mRNA to levels similar to those detected in control BCBL-1 cellular extracts (Fig. 3B). Western immunoblot analysis of BCBL-1 supernatants using anti-VEGF antibody revealed a significant reduction in VEGF secretion when either gB or K8.1 synthesis was inhibited by siRNAs, in agreement with the RT-PCR results described above (Fig. 3C).
Inhibition of gB and K8.1 synthesis causes inhibition of vIL-6 mRNA levels.
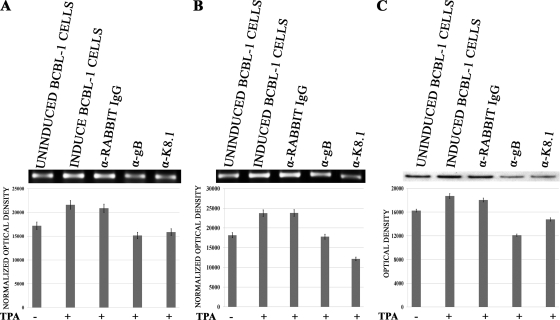
Previous studies have shown that vIL-6 regulated VEGF levels (5, 6). Therefore, similar experiments were performed to determine the role of gB and K8.1 inhibition in vIL-6 mRNA levels. These experiments revealed that the inhibition of either gB or K8.1 synthesis caused a decrease of vIL-6 mRNA levels by 30 to 40%. The combined use of both anti-gB and anti-K8.1 siRNAs did not increase the overall amount of vIL-6 production above that of either the anti-gB or anti-K8.1 siRNAs used alone (Fig. 4). Transfection with plasmid psK5/K8.1co or ps18/gBco restored vIL-6 levels to those detected in mock or nonspecific siRNA control samples (Fig. 4A). To assess whether viral glycoproteins gB and K8.1 in BCBL-1 cell supernatants were involved in the observed transcriptional regulation of VEGF and vIL-6 genes and angiogenesis, BCBL-1 cells were incubated in the presence of anti-gB or anti-K8.1 antibodies during the 24-h TPA induction period. Both anti-gB and K8.1 treatment of BCBL-1 cells caused a significant decrease in both VEGF and vIL-6 transcription and VEGF protein synthesis (Fig. 5).
FIG. 4.
Effect of K8.1 and gB inhibition on vIL-6 production. BCBL-1 cells were transfected with plasmid vectors expressing nonspecific siRNA, ps5, ps5/K8.1co, ps18, ps18/gBco, and ps5/s10 + ps18+ps22. Cells were induced with TPA 24 h posttransfection, and cell pellets were collected 16 h postinduction to extract total RNA and make first-strand cDNA. cDNA from TPA-induced (+) or uninduced (−) BCBL-1 cells and without any plasmids were used as controls. Primers described in Table 1 for vIL-6 amplified a 460-bp DNA fragment, which was visualized by ethidium bromide staining after gel electrophoresis. Bar graphs represent the densitometric analysis of the PCR fragment images using Image J software after normalization to respective GAPDH levels. Experiments were conducted in triplicate, and error bars represent SEM.
FIG. 5.
Effect of anti-K8.1 and anti-gB antibody treatment on VEGF and vIL-6 production. BCBL-1 cells were induced (+) with TPA for 24 h or not induced (−) in the presence of either anti-gB or anti-K8.1 antibody or in the presence of nonspecific rabbit IgG. After 24 h, medium was replaced with serum-free medium and collected after incubation for an additional 16 h. (A) Total RNA was extracted from cell pellets, and the levels of vIL-6 mRNAs were assessed as described in the legend to Fig. 4. (B) The relative levels of VEGF mRNAs also were assessed as described in the legend to Fig. 3. (C) The relative amounts of VEGF protein expressed in supernatants of BCBL-1 cells treated with the various antibodies was assessed by the densitometric analysis of Western immunoblots.
Inhibition of gB and K8.1 synthesis causes substantial reduction in endothelial cell microcapillary formation and migration.
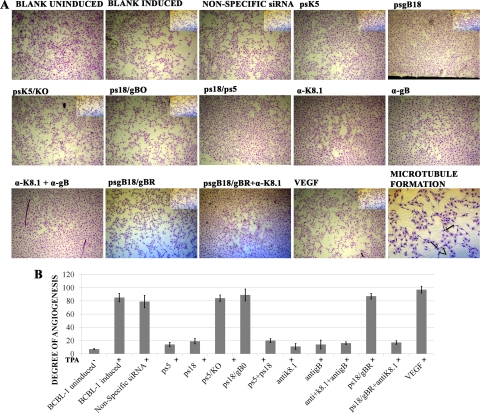
VEGF and vIL-6 are potent promoters of angiogenesis in BCBL-1 tumors in mouse animal models (5, 6). To assess whether the observed reduction of VEGF and vIL-6 mRNA levels affected the behavior of endothelial cells in cell culture, conditioned medium obtained from BCBL-1 cells transfected with anti-gB, anti-K8.1, or a combination of both anti-gB and anti-K8.1 siRNAs was utilized in an in vitro microcapillary formation assay (described in Materials and Methods). These experiments revealed that transfection with siRNAs inhibited the formation of endothelial cell-derived capillary-like tubes in cell culture. Importantly, conditioned medium from BCBL-1 cell cultures treated with the antibodies exhibited a significantly reduced ability to promote angiogenesis, as evidenced by the reduction in the formation of endothelial cell-derived capillary-like tubes (Fig. 6).
FIG. 6.
Effect of K8.1 and gB inhibition on HUVEC microtubule formation. (A) Photomicrographs of HUVEC monolayers treated with conditioned medium obtained from BCBL-1 cells. BCBL-1 cell supernatants were collected as described in Materials and Methods. HUVECs seeded in eight-well chamber slides were treated with equal amounts of total protein from BCBL-1 supernatants for 48 h to allow for microtubule formation. The panel labeled “Microtubule formation” is included to demonstrate the formation of branch points that were counted to obtain the degree of angiogenesis. (B) The degree of angiogenesis was calculated as a product of the total number of branch points and the number branches per point. The branch points were counted under 2× magnification. Magnified portions of the cell cultures are shown as insets. Readings from three random viewing fields were used to generate the bar graphs. Error bars represent SEM.
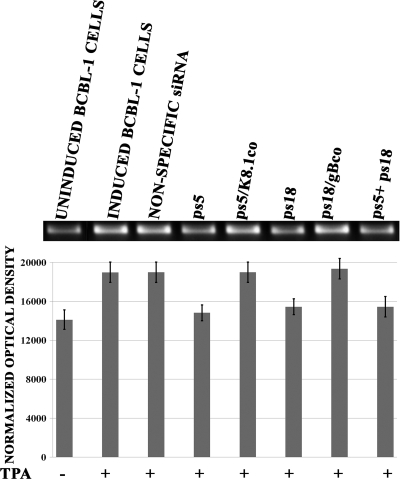
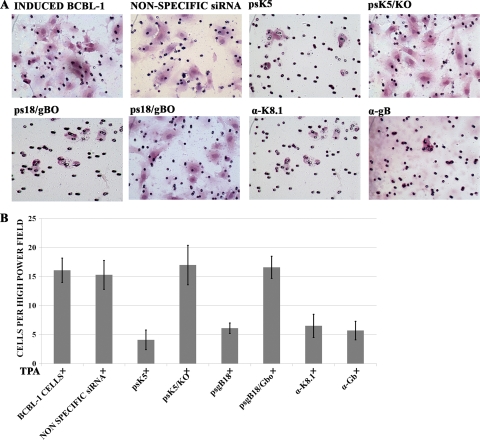
Additional experiments were performed to assess the ability of conditioned medium obtained from BCBL-1 cells subjected to anti-gB or anti-K8.1 siRNA inhibition to induce the migration of HUVEC cells in a Boyden-Chamber migration assay (see Materials and Methods). Conditioned medium obtained from BCBL-1 cells transfected with either anti-gB or anti-K8.1 siRNAs produced significantly reduced cell migration (an approximately 4-fold decrease) compared to that of medium obtained from either TPA-induced, nonspecific siRNA-transfected BCBL-1 cells or a VEGF-positive control sample (Fig. 7).
FIG. 7.
Effect of K8.1 and gB inhibition on HUVEC migration. (A) Photomicrographs of endothelial cell migration toward conditioned medium obtained from BCBL-1 cells. Conditioned medium was collected from BCBL-1 cells treated with either siRNAs or anti-gB and anti-K8.1 antibodies as described in Materials and Methods. Endothelial cells were seeded in the upper chamber of transwell inserts and treated with conditioned medium for 6 h before taking readings. Cells that migrated to the insert's bottom surface were stained with hematoxylin and eosin and counted. (B) Quantification of HUVEC migration. The bar graphs represent the number of cells migrating from the upper chamber to the lower surface of the transwell insert in each treatment. Results are expressed as cells per 40× objective field. Five regions from each of three inserts per condition were averaged. Error bars reflect SEM.
DISCUSSION
KSHV-encoded glycoproteins gB and K8.1 play significant roles in the life cycle of the virus, primarily because they mediate virus attachment to integrin receptors (gB via its RGD motif) and heparan sulfate moieties (gB and K8.1) expressed on cell surfaces. In addition, gB synthesis has been shown to be important for virion egress, implying a specific role of gB in intracellular virion trafficking and egress during the lytic cycle of the virus. Importantly, both gB and gK8.1 have been implicated in signal transduction processes mediated via their binding to integrin and heparan sulfate receptors that most likely contribute to virus-associated pathogenicity. Of particular interest to the present study is the observation that soluble gB and, to a greater extent, K8.1 were shown to induce VEGF synthesis and secretion, suggesting a role of these two viral glycoproteins in angiogenesis and tumorigenesis (55).
The salient features of the results presented in this study are (i) the K8.1 glycoprotein is as important as gB in virion egress; (ii) the gB RGD amino acid motif is not required for virion egress from BCBL-1 cells; and (iii) the inhibition of either gB or K8.1 by siRNAs or via the addition of antibodies to gB or K8.1 into BCBL-1 cultures resulted in a significant reduction in both VEGF and vIL-6 synthesis and the secretion and substantial reduction in the potential of BCBL-1 cells to induce angiogenesis in vitro. These results support the hypothesis that the production of viral particles during lytic reactivation plays an important role in the regulation of VEGF and vIL-6 synthesis via autocrine and/or paracrine signal transduction pathways.
Role of K8.1 and gB in virion egress and infectivity.
We have reported previously that a KSHV recombinant virus carrying a deletion of the K8.1 gene produced infectious virion particles in the supernatants of 293 cells, as evidenced by their ability to enter into 293 cells (30). However, it was noted that the overall amount of infectious virions produced in supernatants of 293 cells was fairly small, which is in part attributed to the fact that these recombinant virions were produced after the transfection of the K8.1 gene-deleted KSHV genome cloned into a bacterial artificial chromosome (66). In the present study, siRNAs against K8.1 were utilized to inhibit K8.1A synthesis in BCBL-1 cells in a manner similar to that of our previous studies examining the role of gB in virion egress (57). The results presented in this work strongly suggest that K8.1 plays a significant role in virion egress that is comparable to that of gB.
The gB RGD motif is known to bind to integrin receptors on cell surfaces mediating virus attachment and virion entry into susceptible cells. We considered that gB synthesized in BCBL-1 cells associates with integrin molecules intracellularly, prior to gB and integrin complex expression on cell surfaces, either in the rough endoplasmic reticulum or within Golgi and trans-Golgi organelles responsible for trafficking both gB and integrin complex protein components on cell surfaces. However, the mutagenesis of the RGD motif showed that there was no effect in virion egress from infected cells, suggesting that the gB RGD motif functions exclusively during virus entry into cells.
Role of gB and K8.1 in angiogenesis.
Generally, the formation of new blood vessels from existing ones is a prerequisite for the growth and metastatic potential of many tumors (15, 16, 20). Tumor and stromal cells are known to secrete two potent angiogenic factors, VEGF and basic fibroblast growth factor (b-FGF) (17, 37). In addition, the physiopathology of hematological malignancies can be affected by these two angiogenic factors. Specifically, a functional linkage between angiogenesis and disease progression has been documented for multiple myelomas (58, 59), non-Hodgkin's lymphoma (49, 52, 53), and leukemia (1, 13, 22, 24, 35, 39, 42).
A number of viral cytokines and chemokines are known to be produced by different primary effusion lymphoma (PEL) cell cultures, including vIL-6 and virally encoded chemokines (vCCLs). The production and secretion of these factors are greatly increased upon the TPA-induced reactivation of the lytic cycle of the virus (29). Similarly, the production and secretion of VEGF and b-FGF are greatly increased upon the lytic reactivation of the virus. Furthermore, the implantation of BCBL-1 and PEL cells in mice resulted in a drastic increase of VEGF and FGF production concomitantly with the detection of reactivated virus (21). Recently, it was shown that the treatment of endothelial cells in cell culture with VEGF increased virus entry into cells, suggesting that the presence of VEGF is a significant factor in virus transmission to proximal and distal sites following primary infection. Similarly, vIL-6 is a key angiogenic and tumorigenic determinant of PEL-like disease in murine models and is known to induce VEGF synthesis in vivo and in vitro (5, 6). Taken together, these results indicate that the spontaneous reactivation of KSHV lytic replication within PEL cells in vivo leads to the production of VEGF and vIL-6 that significantly affects virus-associated pathogenicity by increasing the infectivity of excreted virions and the overall angiogenic and tumorigenic potential of PEL tumors.
It has been shown previously that soluble versions of either gB or K8.1 appear to regulate VEGF-A synthesis (55). However, the fact that gB having its RGD motif changed to RAA rescued virion egress and both VEGF and vIL-6 production argues against the involvement of a gB-mediated, integrin-dependent signal transduction pathway in VEGF/vIL-6 regulation. The fact that the simultaneous use of anti-gB and anti-K8.1 siRNAs did not reduce VEGF/vIL-6 production by levels greater than those observed when either gB or K8.1 was targeted alone suggests that gB and K8.1 act via the same mechanistic pathway. In this regard, it is possible that gB directly or indirectly interacts and signals as a protein complex with K8.1, explaining the ability of the RGD-modified gB to rescue VEGF and vIL-6 synthesis. The anti-gB and anti-K8.1 antibody treatment experiments clearly show that gB and K8.1 found in supernatants and/or infected cell surfaces are responsible for the VEGF and vIL-6 regulation.
Our mechanistic model for explaining the observed phenomena suggests a direct interaction between gB and K8.1 in virion particles and cell surfaces and their interaction with one or more plasma membrane receptors that mediate VEGF and vIL-6 transcriptional regulation. These transduction receptors may include the known αVβ3 and xCT KSHV receptors as well as the vIL-6 receptor. Finally, gB and K8.1 are not efficiently secreted in supernatants of BCBL-1 cells as free proteins, suggesting that most gB and K8.1 is found either in virion particles or stably immobilized on BCBL-1 cell surfaces. Based on the observations that BCBL-1 cells do not efficiently aggregate, it can be concluded that virion particles are primarily responsible for the observed regulation of VEGF and vIL-6 synthesis. Therefore, secreted virion particles play direct and important roles in angiogenesis. It has been noted that the KSHV infection of endothelial cells induces a number of other angiogenic and inflammatory cytokines, such as Ang-2, MMP1, MMP9, and IL-6 (47, 48, 64, 65). Most likely, these proteins are upregulated via intracellular pathways that are altered as a result of KSHV infection. The results presented herein suggest that the targeted inhibition of gB and/or K8.1 synthesis reduces the pathogenicity, angiogenicity, and tumorigenicity of the virus in vivo.
Acknowledgments
This work was supported by a subproject of grant NIH:NCRR P20 RR16456 to O.D., NIH:NIAID AI43000 to K.G.K., and core facilities of NIH NCRR P20 RR020159 to K.G.K.
We gratefully acknowledge the BIOMMED staff, especially Jason Walker for proofreading the document.
Footnotes
Published ahead of print on 2 December 2009.
REFERENCES
- 1.Aguayo, A., H. Kantarjian, T. Manshouri, C. Gidel, E. Estey, D. Thomas, C. Koller, Z. Estrov, S. O'Brien, M. Keating, E. Freireich, and M. Albitar. 2000. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 96:2240-2245. [PubMed] [Google Scholar]
- 2.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 3.Akula, S. M., F. Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. [DOI] [PubMed] [Google Scholar]
- 4.Antman, K., and Y. Chang. 2000. Kaposi's sarcoma. N Engl. J. Med. 342:1027-1038. [DOI] [PubMed] [Google Scholar]
- 5.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 6.Aoki, Y., and G. Tosato. 1999. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood 94:4247-4254. [PubMed] [Google Scholar]
- 7.Baghian, A., M. Luftig, J. B. Black, Y. X. Meng, C. P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. [DOI] [PubMed] [Google Scholar]
- 8.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 9.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, W. Z., S. Person, S. C. Warner, J. H. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandran, B., C. Bloomer, S. R. Chan, L. Zhu, E. Goldstein, and R. Horvat. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249:140-149. [DOI] [PubMed] [Google Scholar]
- 13.Chen, H., A. T. Treweeke, D. C. West, K. J. Till, J. C. Cawley, M. Zuzel, and C. H. Toh. 2000. In vitro and in vivo production of vascular endothelial growth factor by chronic lymphocytic leukemia cells. Blood 96:3181-3187. [PubMed] [Google Scholar]
- 14.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, et al. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 5:3057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folkman, J. 1971. Tumor angiogenesis: therapeutic implications. N Engl. J. Med. 285:1182-1186. [DOI] [PubMed] [Google Scholar]
- 16.Folkman, J., K. Watson, D. Ingber, and D. Hanahan. 1989. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 339:58-61. [DOI] [PubMed] [Google Scholar]
- 17.Friesel, R. E., and T. Maciag. 1995. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J. 9:919-925. [DOI] [PubMed] [Google Scholar]
- 18.Ganem, D. 1998. Human herpesvirus 8 and its role in the genesis of Kaposi's sarcoma. Curr. Clin. Top. Infect. Dis. 18:237-251. [PubMed] [Google Scholar]
- 19.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin alphaVbeta3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 82:1570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gimbrone, M. A., Jr., S. B. Leapman, R. S. Cotran, and J. Folkman. 1972. Tumor dormancy in vivo by prevention of neovascularization. J. Exp. Med. 136:261-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haddad, L., H. El Hajj, R. Abou-Merhi, Y. Kfoury, R. Mahieux, M. El-Sabban, and A. Bazarbachi. 2008. KSHV-transformed primary effusion lymphoma cells induce a VEGF-dependent angiogenesis and establish functional gap junctions with endothelial cells. Leukemia 22:826-834. [DOI] [PubMed] [Google Scholar]
- 22.Hussong, J. W., G. M. Rodgers, and P. J. Shami. 2000. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 95:309-313. [PubMed] [Google Scholar]
- 23.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 24.Kini, A. R., N. E. Kay, and L. C. Peterson. 2000. Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia 14:1414-1418. [DOI] [PubMed] [Google Scholar]
- 25.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyano, S., E. C. Mar, F. R. Stamey, and N. Inoue. 2003. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 84:1485-1491. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan, H. H., N. Sharma-Walia, L. Zeng, S. J. Gao, and B. Chandran. 2005. Envelope glycoprotein gB of Kaposi's sarcoma-associated herpesvirus is essential for egress from infected cells. J. Virol. 79:10952-10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lake, C. M., and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus that lacks glycoprotein gN is impaired in assembly and infection. J. Virol. 74:11162-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, C., Y. Okruzhnov, H. Li, and J. Nicholas. 2001. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 75:10933-10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luna, R. E., F. Zhou, A. Baghian, V. Chouljenko, B. Forghani, S. J. Gao, and K. G. Kousoulas. 2004. Kaposi's sarcoma-associated herpesvirus glycoprotein K8.1 is dispensable for virus entry. J. Virol. 78:6389-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maheshwari, R. K., V. Srikantan, D. Bhartiya, H. K. Kleinman, and D. S. Grant. 1991. Differential effects of interferon gamma and alpha on in vitro model of angiogenesis. J. Cell. Physiol. 146:164-169. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy, J. B., S. L. Palm, and L. T. Furcht. 1983. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J. Cell Biol. 97:772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittal, V. 2004. Improving the efficiency of RNA interference in mammals. Nat. Rev. Genet. 5:355-365. [DOI] [PubMed] [Google Scholar]
- 34.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 35.Molica, S., A. Vacca, D. Ribatti, A. Cuneo, F. Cavazzini, D. Levato, G. Vitelli, L. Tucci, A. M. Roccaro, and F. Dammacco. 2002. Prognostic value of enhanced bone marrow angiogenesis in early B-cell chronic lymphocytic leukemia. Blood 100:3344-3351. [DOI] [PubMed] [Google Scholar]
- 36.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neufeld, G., T. Cohen, S. Gengrinovitch, and Z. Poltorak. 1999. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13:9-22. [PubMed] [Google Scholar]
- 38.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padró, T., S. Ruiz, R. Bieker, H. Burger, M. Steins, J. Kienast, T. Buchner, W. E. Berdel, and R. M. Mesters. 2000. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood 95:2637-2644. [PubMed] [Google Scholar]
- 40.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira, L. 1994. Function of glycoprotein B homologues of the family herpesviridae. Infect. Agents Dis. 3:9-28. [PubMed] [Google Scholar]
- 42.Perez-Atayde, A. R., S. E. Sallan, U. Tedrow, S. Connors, E. Allred, and J. Folkman. 1997. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am. J. Pathol. 150:815-821. [PMC free article] [PubMed] [Google Scholar]
- 43.Perry, S. T., and T. Compton. 2006. Kaposi's sarcoma-associated herpesvirus virions inhibit interferon responses induced by envelope glycoprotein gpK8.1. J. Virol. 80:11105-11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 76:4390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 46.Pertel, P. E., P. G. Spear, and R. Longnecker. 1998. Human herpesvirus-8 glycoprotein B interacts with Epstein-Barr virus (EBV) glycoprotein 110 but fails to complement the infectivity of EBV mutants. Virology 251:402-413. [DOI] [PubMed] [Google Scholar]
- 47.Qian, L. W., W. Greene, F. Ye, and S. J. Gao. 2008. Kaposi's sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of VE-cadherin. J. Virol. 82:11902-11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian, L. W., J. Xie, F. Ye, and S. J. Gao. 2007. Kaposi's sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J. Virol. 81:7001-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ribatti, D., A. Vacca, B. Nico, M. Fanelli, L. Roncali, and F. Dammacco. 1996. Angiogenesis spectrum in the stroma of B-cell non-Hodgkin's lymphomas. An immunohistochemical and ultrastructural study. Eur. J. Haematol. 56:45-53. [DOI] [PubMed] [Google Scholar]
- 50.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 51.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salven, P., A. Orpana, L. Teerenhovi, and H. Joensuu. 2000. Simultaneous elevation in the serum concentrations of the angiogenic growth factors VEGF and bFGF is an independent predictor of poor prognosis in non-Hodgkin lymphoma: a single-institution study of 200 patients. Blood 96:3712-3718. [PubMed] [Google Scholar]
- 53.Salven, P., L. Teerenhovi, and H. Joensuu. 1997. A high pretreatment serum vascular endothelial growth factor concentration is associated with poor outcome in non-Hodgkin's lymphoma. Blood 90:3167-3172. [PubMed] [Google Scholar]
- 54.Schulz, T. F., J. Sheldon, and J. Greensill. 2002. Kaposi's sarcoma associated herpesvirus (KSHV) or human herpesvirus 8 (HHV8). Virus Res. 82:115-126. [DOI] [PubMed] [Google Scholar]
- 55.Sivakumar, R., N. Sharma-Walia, H. Raghu, M. V. Veettil, S. Sadagopan, V. Bottero, L. Varga, R. Levine, and B. Chandran. 2008. Kaposi's sarcoma-associated herpesvirus induces sustained levels of vascular endothelial growth factors A and C early during in vitro infection of human microvascular dermal endothelial cells: biological implications. J. Virol. 82:1759-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 57.Subramanian, R., O. D'Auvergne, H. Kong, and K. G. Kousoulas. 2008. The cytoplasmic terminus of Kaposi's sarcoma-associated herpesvirus glycoprotein B is not essential for virion egress and infectivity. J. Virol. 82:7144-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vacca, A., D. Ribatti, M. Presta, M. Minischetti, M. Iurlaro, R. Ria, A. Albini, F. Bussolino, and F. Dammacco. 1999. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 93:3064-3073. [PubMed] [Google Scholar]
- 59.Vacca, A., D. Ribatti, L. Roncali, G. Ranieri, G. Serio, F. Silvestris, and F. Dammacco. 1994. Bone marrow angiogenesis and progression in multiple myeloma. Br. J. Haematol. 87:503-508. [DOI] [PubMed] [Google Scholar]
- 60.Veettil, M. V., S. Sadagopan, N. Sharma-Walia, F. Z. Wang, H. Raghu, L. Varga, and B. Chandran. 2008. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (alphaVbeta5, alphaVbeta3, and alpha3beta1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J. Virol. 82:12126-12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vieira, J., P. O'Hearn, L. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 75:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 75:7517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, L., N. Wakisaka, C. C. Tomlinson, S. M. DeWire, S. Krall, J. S. Pagano, and B. Damania. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 64:2774-2781. [DOI] [PubMed] [Google Scholar]
- 64.Xie, J., H. Pan, S. Yoo, and S. J. Gao. 2005. Kaposi's sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J. Virol. 79:15027-15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye, F. C., D. J. Blackbourn, M. Mengel, J. P. Xie, L. W. Qian, W. Greene, I. T. Yeh, D. Graham, and S. J. Gao. 2007. Kaposi's sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J. Virol. 81:3980-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu, L., V. Puri, and B. Chandran. 1999. Characterization of human herpesvirus-8 K8.1A/B glycoproteins by monoclonal antibodies. Virology 262:237-249. [DOI] [PubMed] [Google Scholar]
- 68.Zhu, L., R. Wang, A. Sweat, E. Goldstein, R. Horvat, and B. Chandran. 1999. Comparison of human sera reactivities in immunoblots with recombinant human herpesvirus (HHV)-8 proteins associated with the latent (ORF73) and lytic (ORFs 65, K8.1A, and K8.1B) replicative cycles and in immunofluorescence assays with HHV-8-infected BCBL-1 cells. Virology 256:381-392. [DOI] [PubMed] [Google Scholar]