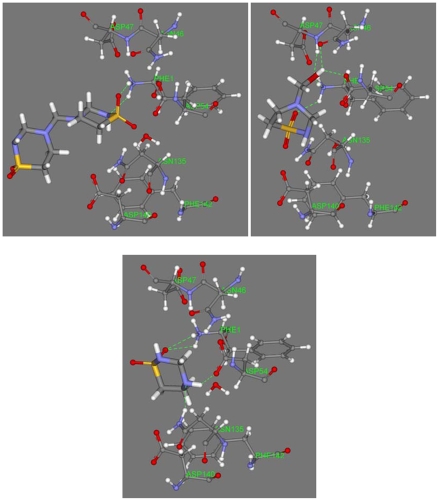
Figure 11. Stick display of taurolidine and its derivatives, methylol taurultam and taurultam, at the active site of E. coli FimH.
Top left: taurolidine with H-bond interacting amino acids and water (ball and spoke display) shows the formation of a 6-membered ring -H-N(phe1)-H-O(sulfonyl)-S-N(taur)-; the non interacting taurolidine ring protrudes outside the protein pocket. Top right: methylol taurultam shows 4 H-bonds: (a) S(sulfonyl) as a hydrogen bond acceptor from the NH3-(Phe1), (b) O–H acts as a bifurcated hydrogen bond donor to O(carbonyl) of Arg46 and Phe1, (c) O(hydroxyl)as a hydrogen bond acceptor from HN-(Asp47). Bottom: taurultam also has 4 hydrogen bonds: (a) O(sulfonyl) as a hydrogen bond acceptor from two hydrogens of NH3-(Phe1), (b) N-H as hydrogen bond donor to O(carbonyl)-(Asp54) and (c) hydrogen bond acceptor from H-N-(Asn135).