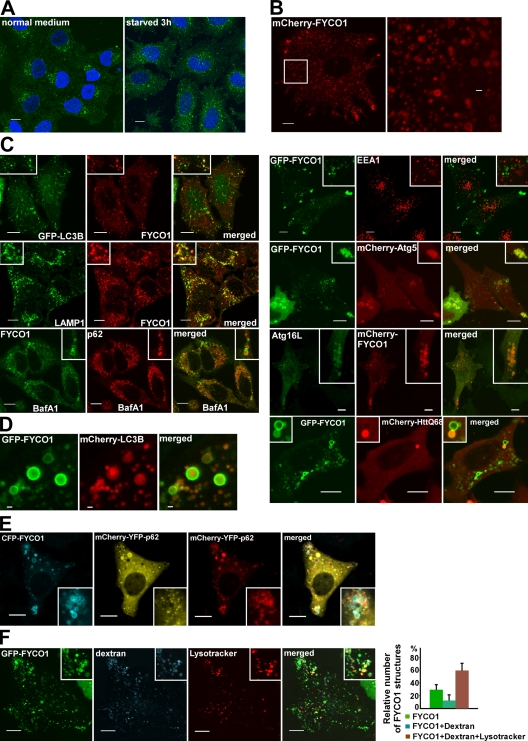
Figure 3.
FYCO1 decorates cytosolic vesicles and cytosolic punctuated structures containing the protein markers of autophagosomes/LEs/lysosomes. (A) Endogenous FYCO1 localizes to the cytosolic punctuated structures. HeLa cells cultured in complete growth medium (left) or in HBSS for 3 h (right) were fixed and stained with anti-FYCO1 antibody (green) and Draq5 (blue). (B) Overexpressed mCherry-FYCO1 localizes to the cytosolic punctuated structures and cytosolic vesicles in stably transfected HeLa cells. (C) FYCO1 partially colocalizes with LC3B, LAMP1, ATG5, ATG16L, p62, and HuntingtinQ64 and does not colocalize with EEA1. HeLa cells or mouse embryonic fibroblasts (for ATG16L staining) were transfected with the indicated vectors or stained with the indicated antibodies and imaged 24 h after transfection. For p62 staining, HeLa cells were incubated with 0.2 µM BafA1 for 12 h before fixation. (D) FYCO1 colocalizes with LC3B on the rim but not inside of the vesical structures. HeLa cells cotransfected with GFP-FYCO1, mCherry-LC3B, and myc-p62 were imaged by confocal microscopy 48 h after transfection. (E and F) FYCO1-decorated structures are heterogenous in their luminal content. (E) HeLa cells transfected with CFP-FYCO1 and mChery-YFP-p62 were imaged 48 h after transfection. (F) HeLa cells transfected with GFP-FYCO1 were labeled with LysoTracker red for 60 min and Alexa Fluor 647–dextran (10,000 D) for 4 h in normal growth medium to stain late endocytic compartments (left). Relative number of FYCO1 structures ± SD colocalized with LysoTracker red or Alexa Fluor 647–dextran (10,000 D; right). (B, C, E, and F) Insets show an enlarged field of interest. Bars: (A, B [left], C, E, and F) 10 µm; (B [right] and D) 1 µm.