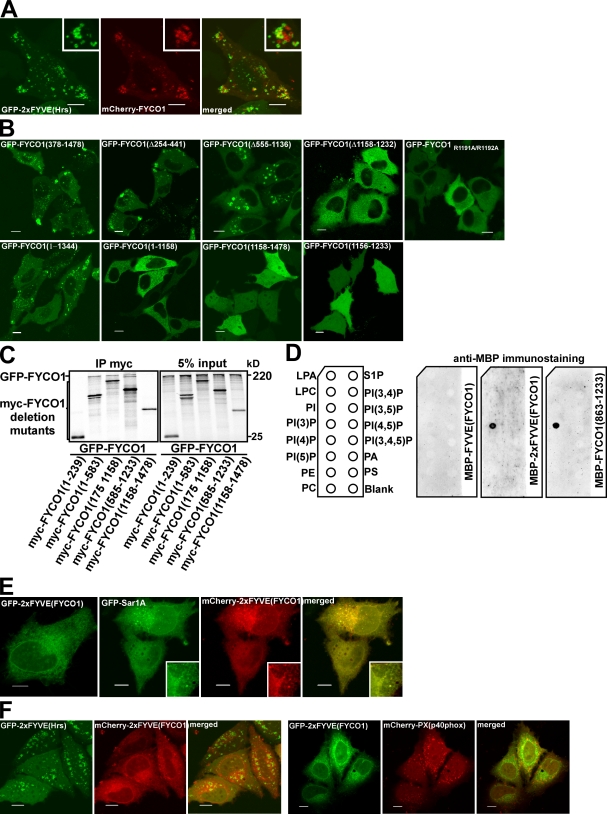
Figure 5.
FYCO1 can dimerize and is recruited to intracellular membranes in an FYVE domain–dependent manner. (A) FYCO1 shows only weak or no colocalization with a PI3P probe 2xFYVEHrs. HeLa cells were imaged 24 h after transfection with the indicated constructs. (B) The FYVE domain of FYCO1 is essential but not sufficient for its recruitment to the cytosolic vesicles. HeLa cells were imaged 24 h after transfection with the indicated constructs. (C) FYCO1 can self-interact in a CC-dependent manner. Full-length GFP-FYCO1 was cotranscribed and cotranslated with the indicated deletion mutants of myc-tagged FYCO1 in rabbit reticulocyte lysate. S35-labeled FYCO1 complexes were immunoprecipitated with anti-myc antibody, separated by SDS-PAGE, and visualized by autoradiography. IP, immunoprecipitation. (D) Dimers but not a monomer of the FYVE domain from FYCO1 can bind PI3P in protein–lipid overlay assay. PIP Strips were incubated with 1-µg/ml solutions of MBP-FYVEFYCO1, MBP-2xFYVEFYCO1, or MBP-FYCO1863–1,233 for 1 h, and bound proteins were detected by immunostaining with anti-MBP antibody. LPA, lysophosphatic acid; LPC, lysophosphocholine; PI, phosphatidylinositol; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine-1-phosphate; PA, phosphatidic acid; PS, phosphatidylserine. (E) The construct containing a tandem fusion of two FYVE domains from FYCO1 (2xFYVEFYCO1) localizes to the nuclear envelope and ER in HeLa cells. HeLa cells were imaged 24 h after transfection with the indicated constructs. (A and E) Insets show an enlarged field of interest. (F) 2xFYVEFYCO1 does not colocalize with 2xFYVEHrs (left) and PXp40phox (right). HeLa cells were imaged 24 h after transfection with the indicated constructs. Bars, 10 µm.