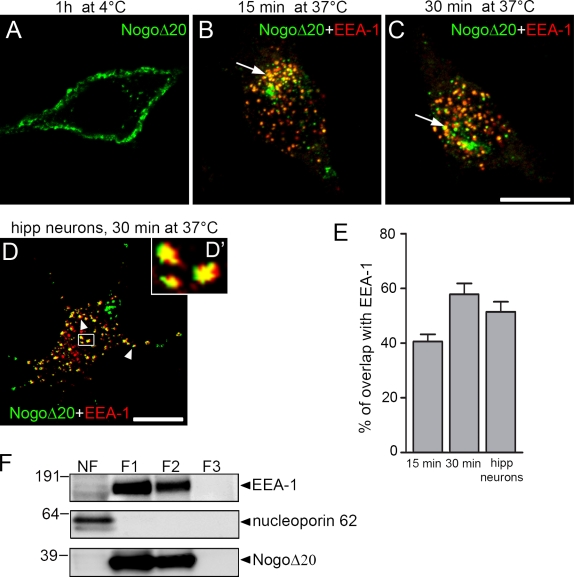
Figure 1.
Surface binding and internalization of NogoΔ20 in neuronal cells. (A–C) Representative confocal immunofluorescence optical sections of PC12 cells that were incubated with 300 nM NogoΔ20-T7 for 1 h at 4°C (A), or for 15 min (B) or 30 min (C) at 37°C. Cells were stained with anti-T7 mAb for NogoΔ20 (green) and with anti–EEA-1 as an early endosomal marker (red), and analyzed by confocal microscopy. Arrows indicate colocalization of NogoΔ20 with EEA-1. Bar, 10 µm. (D) Dissociated hippocampal neurons cultured for 4 d were incubated with 300 nM NogoΔ20-T7 for 30 min at 37°. NogoΔ20 (green) appears in vesicular structures in the cell body and neurites of the neurons, as indicated with arrowheads. NogoΔ20-positive vesicles colocalize with EEA-1 (red). The inset panel shows an enlarged view of the boxed region. Bar, 10 µm. (E) Quantification of colocalization of NogoΔ20 with EEA-1 after indicated time points. Values are given as the mean from three independent experiments ± SEM. (F) Subcellular fractionation of PC12 cells after internalization of NogoΔ20-T7 for 30 min at 37°C. Cells were homogenized and centrifuged to separate nuclei (NF, nuclear fraction) from the postnuclear supernatant, which was loaded on a 8–40.6% sucrose step gradient to separate different organelles. After centrifugation, different fractions F1 (8–25% sucrose), F2 (25–35% sucrose), and F3 (35–40.6% sucrose) were collected and immunoblotted for EEA-1 (top), nucleoporin p62 (middle), and NogoΔ20-T7 (bottom). A representative blot from three experiments is shown.