Abstract
Vascular and neuronal networks share a similar branching morphology, and emerging evidence implicates common mechanisms in the formation of both systems. δ-Catenin is considered a neuronal catenin regulating neuron cell–cell adhesion and cell motility. Here, we report expression of δ-catenin in vascular endothelium, and show that deletion of only one allele of δ-catenin is sufficient to impair endothelial cell motility and vascular assembly in vitro and pathological angiogenesis in vivo, thereby inhibiting tumor growth and wound healing. In contrast, deletion of one or both allele of δ-catenin had no effects on hormone-induced physiological angiogenesis in the uterus. Molecular analysis confirmed a gene dosage effect of δ-catenin on Rho GTPase activity. Moreover, we show that inflammatory cytokines, but not angiogenic factors, regulate δ-catenin expression, and the levels of δ-catenin positively correlate to human lung cancers. Collectively, our data suggest that inflammation, commonly associated with disease conditions, induces δ-catenin expression that specifically regulates pathological, and not physiological, angiogenesis. Because only pathological angiogenesis is sensitive to decreased levels of δ-catenin, this may provide a good target for antiangiogenic therapy.
Vascular network formation is essential for tissue growth during development. When we reach adulthood, the vascular endothelium becomes quiescent. However, under disease conditions, this delicate balance is disturbed, and endothelium is reactivated. Knowing how pathological angiogenesis occurs has important implications in controlling the growth and progression of diseases such as cancer.
The vascular system shares several striking anatomical similarities with the nervous system. They both consist of a highly complex and precisely branched network. They often run parallel and intimately interact, suggesting a common mechanism regulating the formation of both networks (Mukouyama et al., 2002; Weinstein, 2005; Carmeliet and Tessier-Lavigne, 2005; Jones and Li, 2007). Indeed, findings have shown that angiogenic factors, such as VEGF, also regulate neuronal growth and development (Oosthuyse et al., 2001; Jin et al., 2002). Reciprocally, neuropilin, a receptor for semaphorin that controls neuronal growth, is also expressed in vascular endothelium and regulates vascular development (Lee et al., 2002).
δ-Catenin is a neuronal protein containing 10 Armadillo repeats. It belongs to the p120 family of proteins (Hatzfeld, 2005). The major functions of the p120 protein family include stabilization of cadherins by binding to a highly conserved sequence in the juxtamembrane region, as well as regulating Rho GTPase activity and cell motility (Reynolds, 2007). δ-Catenin is localized to the postsynaptic adherens junction in neuronal cells, collaborates with Rho GTPases to set a balance between neurite elongation and branching, and induces dendritic protrusions in neurons (Martinez et al., 2003). Genetic deletion of δ-catenin in mice results in severe deficits in several types of memory and synaptic plasticity (Israely et al., 2004), which is consistent with the neuronal phenotype.
In this study, we demonstrate haploinsufficiency of δ-catenin specifically in pathological angiogenesis. δ-Catenin is expressed in vascular endothelium. It regulates endothelial cell motility and angiogenesis in tumors and wound healing in a gene dosage–sensitive manner. In addition, inflammatory cytokines up-regulate the gene expression and the levels of δ-catenin are elevated in human lung cancers compared with surrounding normal tissues, which is consistent with its role in pathological angiogenesis, as inflammation is closely associated with cancer.
RESULTS
Neuronal δ-catenin is expressed in vascular endothelium
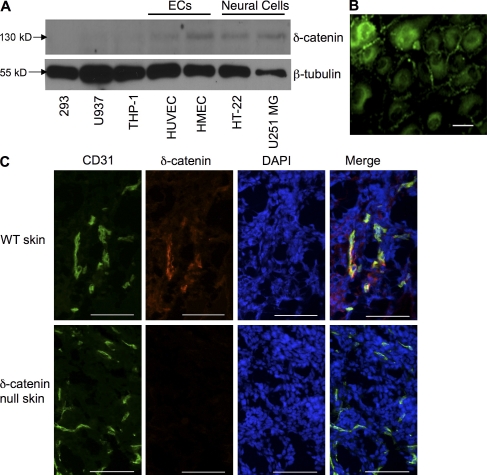
Based on the observation that the vascular and neuronal systems share a similar treelike branched structure and certain common mediators (Jones and Li, 2007), we postulate that δ-catenin, a neuronal catenin, may have a role in vascular endothelium. To test this hypothesis, we examined δ-catenin expression in human endothelial cells. We included neuronal cells (HT-22 and U251MG), as well as epithelial cells (293) and leukocytes (U937 and THP-1). In addition to neuronal cell lines, δ-catenin expression was clearly demonstrated in large (human umbilical vein endothelial cell [HUVEC]) and small (human microvascular endothelial cell [HMEC]) vascular endothelial cells, but not in any of the other cell types tested (Fig. 1 A). Immunofluorescent staining in cultured monolayer endothelial cells demonstrated δ-catenin expression at cell–cell junctions and intracellularly (Fig. 1 B), consistent with the function of δ-catenin in cell–cell junctions and cell motility.
Figure 1.
δ-Catenin is expressed in vascular endothelium. (A) Protein lysates collected from neuronal cells (HT-22 and U251 MG), endothelial cells (ECs; HUVEC and HMEC), epithelial cells (293), and leukocytes (THP-1 and U937) were subjected to Western blot analysis and probed with a δ-catenin–specific antibody. (B) β-tubulin was used as a loading control. Immunofluorescent staining for δ-catenin was performed in cultured HUVECs. (C) Mouse skin tissue sections from wild-type and δ-catenin–null mice were analyzed by immunofluorescent double staining using antibodies against CD31 for endothelium, and δ-catenin. Nuclei were stained with DAPI. 400× magnification. Each experiment was repeated three times, and representative images were shown. Bar, 200 µm.
Additionally, we analyzed expression of δ-catenin in tissue samples. A double staining using antibodies against δ-catenin and CD31, a marker for endothelium, confirmed expression of the gene in vascular endothelium in skin (Fig. 1 C) and tumor samples (Fig. S1). The δ-catenin antibody failed to detect any signal in tissues harvested from δ-catenin–null mice (Fig. 1 C and Fig. S1). Collectively, these findings for the first time demonstrate the presence of neuronal δ-catenin in vascular endothelium. Considering the role of δ-catenin in cell–cell junction and cell motility, these data suggest a role for δ-catenin in angiogenesis.
δ-Catenin regulates endothelial cell motility and vascular network formation in a gene dosage–dependent manner in vitro
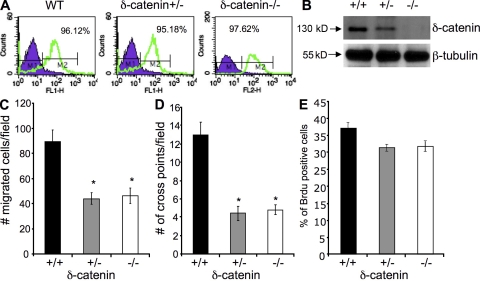
To test the function of δ-catenin in angiogenesis, we used δ-catenin–null mice. Besides deficiency in memory and synaptic plasticity, the null mice are healthy, fertile, and they grow normally (Israely et al., 2004). We isolated and purified microvascular endothelial cells from lungs of wild-type, heterozygous, and δ-catenin knockout mice. We achieved >95% purity (CD31 positive) in each group of cells (Fig. 2 A). Reduction and total absence of δ-catenin expression in heterozygous- and homozygous-null cells, respectively, were confirmed by Western blot (Fig. 2 B). Next, we analyzed the role of δ-catenin in angiogenesis by measuring endothelial cell motility, vascular sprouting in a 3-D Matrigel assay, and cell proliferation. We found that a total deletion of δ-catenin significantly impaired cell motility and vascular structure formation, but deletion of δ-catenin had no effects on cell proliferation (Fig. 2, C–E). Surprisingly, we detected δ-catenin haploinsufficiency in regulating angiogenesis. There is a significant reduction of cell motility and vascular structure formation when deleting only one allele of the gene (Fig. 2, C–E). The levels of angiogenic function in heterozygous cells are similar to the ones from homozygous-null cells. These findings reveal new functions of δ-catenin in angiogenesis that act in a gene dosage–dependent manner.
Figure 2.
δ-Catenin regulates endothelial cell motility and vascular tubule formation in vitro in a gene dosage–sensitive manner. (A) Microvascular endothelial cells were isolated and pooled from lungs (5 mice per group) of wild-type littermates, δ-catenin heterozygous-null, and homozygous-null mice. The endothelial cells were sorted with a CD31 antibody with a FACStarPlus flow cytometer. The percentage of CD31 positive cells was indicated in each graph. (B) The levels of δ-catenin in wild-type, δ-catenin+/−, and δ-catenin−/− endothelial cells were evaluated in cell lysates by Western blot. Endothelial cell migration and vascular tubule formation were measured in Transwell assay or Matrigel assay, respectively. (C) Migrated cells were counted after a 5-h incubation in 10 randomly selected high-power fields under microscopy. The data were collected from three independent experiments. Mean and SE were plotted. *, P < 0.01. (D) Vascular tubule formation was measured 18 h after cell plating. Vascular cross points were counted from 10 randomly selected high-power fields under microscopy. The data were collected from three independent experiments. *, P < 0.01. (E) Cell proliferation was measured by BrdU incorporation. The experiment was done in duplicate and repeated three times.
To verify these findings, we also applied short hairpin RNA (shRNA) to specifically knockdown δ-catenin in human endothelial cells. HUVECs were treated with shRNA for 24 h, and expression levels of δ-catenin were verified using Western blot. We achieved ∼75% knockdown of δ-catenin expression in endothelial cells compared with controls (Fig. S2 A). Consistently, knockdown of δ-catenin in endothelial cells significantly impaired cell motility (Fig. S2 B) and vascular tubule formation in 3-D Matrigel (Fig. S2 C), but had no effect on cell proliferation (not depicted). Reciprocally, overexpression of δ-catenin in endothelial cells using an expression vector (Fig. S2 D) significantly increased cell motility (Fig. S2 E) and vascular network formation in Matrigel (Fig. S2 F). Collectively, these data support a role of δ-catenin in angiogenesis via regulation of endothelial cell motility and vascular network assembly.
δ-Catenin inhibits RhoA activity, but enhances Rac1 and Cdc42 activity, likely through interaction with Vav1 in endothelial cells
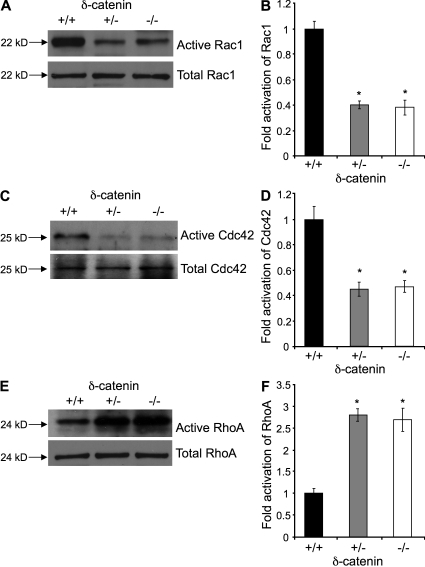
The p120 family of proteins, including δ-catenin, is known to regulate cell motility through Rho GTPases (Noren et al., 2000; Grosheva et al., 2001). We therefore determined the effects of δ-catenin on Rho GTPase activation in endothelial cells isolated from wild-type, heterozygous-null, and homozygous-null mice. Consistent with the observation of haploinsufficiency of δ-catenin in angiogenic functions, we found that reduction or deletion of the δ-catenin gene significantly inhibited activation of Rac1 and Cdc42, but enhanced RhoA activation (Fig. 3), Similar observations were made with δ-catenin knockdown in human endothelial cells, and the opposite was observed with overexpression of δ-catenin (Fig. S3). These findings provide molecular mechanism for haploinsufficiency of δ-catenin in endothelial cell motility and angiogenesis.
Figure 3.
δ-Catenin regulates Rho GTPase activity in a gene dosage sensitive manner. Microvascular endothelial cells isolated from wild-type littermates, δ-catenin heterozygous-null, and homozygous-null mice were analyzed for Rho GTPase activity. Activation of Rac1 and Cdc42 was measured by PAK1-efficient pull-down assays. Precipitated activated Rac1 (A and B) or Cdc42 (C and D) and each total protein from cell lysate were analyzed by Western blot with specific antibody. RhoA activation was pulled down with Rhotekin-agarose beads, analyzed by Western blot, and probed with a RhoA-specific antibody (E and F). *, P < 0.001. Each experiment was repeated three times, and representative images were shown.
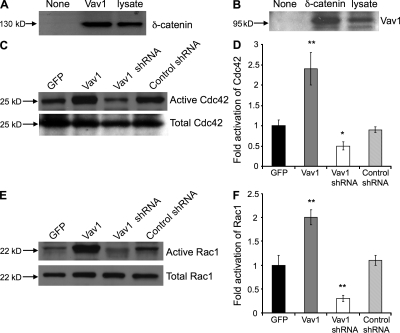
Vav1 is a guanine nucleotide exchange factor for Rho GTPases. Vav1 is specifically expressed in hematopoietic cells (Bustelo, 2000; Tybulewicz et al., 2003), as well as in endothelial cells (Georgiades et al., 2002). Using immunoprecipitation, we demonstrated an association of Vav1 with δ-catenin in cultured endothelial cells (Fig. 4, A and B). Furthermore, overexpression of Vav1 in endothelial cells significantly increased Cdc42 and Rac1 activation (Fig. 4, C–F). Reciprocally, knockdown of Vav1 by shRNA led to a significant reduction of activation of these two Rho GTPases. All together, these results indicate that δ-catenin mediates Rac1 and Cdc42 activation in endothelial cells likely through Vav1.
Figure 4.
Vav1 interacts with δ-catenin and activates Cdc42 and Rac1. HUVECs were transfected with either an expression vector for control GFP or δ-catenin, or transfected with control shRNA or δ-catenin shRNA for 48 h. (A) Cell lysate was immunoprecipitated with antibody against Vav1 or control beads (none), and subjected to Western blot analysis with antibody against δ-catenin. Equal amount of cell lysate (lysate) was used as a loading control. (B) Cell lysate was also immunoprecipitated with antibody against δ-catenin or control beads (none) and subjected to Western blot analysis for Vav1. Equal amount of lysate (lysate) was used as a loading control. Cell lysates were analyzed for Cdc42 (C) and Rac1 (E) activation using PAK1-efficient pull-down assay. Precipitated and total protein was analyzed by Western blot. The levels of activated Cdc42 were compared with total levels of Cdc42 (D), and activated Rac1 to total levels of Rac1 (F). *, P < 0.05 and **, P < 0.01, compared with corresponding control. Each experiment was repeated three times, and representative images were shown.
Haploinsufficiency of δ-catenin impairs pathological angiogenesis in tumor growth and wound healing
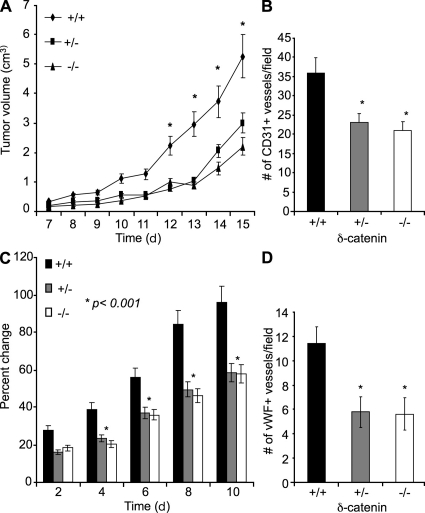
As tumor growth depends on tumor angiogenesis, we therefore investigated the function of δ-catenin in tumor angiogenesis. Murine 3LL tumor cells were implanted in syngeneic δ-catenin homozygous-null, heterozygous-null, and wild-type littermates. Tumor growth was measured over time. Consistent with the in vitro data on gene dosage of δ-catenin in angiogenesis, we observed a significant retardation in tumor growth in homozygous-null mice, as well as in heterozygous-null mice, compared with wild-type controls (Fig. 5 A), which correlates to a reduced tumor vascular density in tumors harvested from these null mice (Fig. 5 B and Fig. S4). No significant difference in tumor growth and tumor vascular density between the heterozygous- and homozygous-null mice was observed, consistent with the observations of endothelial cell motility and vascular structure formation between these two groups. The experiment was repeated twice, and consistent results were achieved.
Figure 5.
Genetic deletion of δ-catenin in mice impairs pathological angiogenesis in a dose-dependent manner. 3LL tumor cells were injected subcutaneously into sex- and age-matched syngeneic δ-catenin homozygous-null, heterozygous-null, and wild-type littermates. (A) Tumor growth was measured by caliper for 15 d, and tumor volume was calculated and plotted (n = 10 mice/group; *, P < 0.001). (B) Tumor tissue sections from each group were analyzed by CD31 immunofluorescent staining, and CD31 positive vessels were counted from 10 randomly selected high-power fields under microscopy. Mean and SE were plotted. *, P < 0.05. (C) 6-mm biopsy punches were made in wild-type littermates, δ-catenin heterozygous-null, and δ-catenin homozygous-null mice. Wound size was measured daily. The percent changes of wound size over the original size were plotted (n = 7 mice/group; *, P < 0.01). (D) The vascular density measurement by vWF staining was performed in wounded tissues on day 3. Mean and SE were plotted. *, P < 0.01.
To further establish the role of δ-catenin in angiogenesis in vivo, we also tested it in wound healing using a skin biopsy puncture model. Consistent with the results from tumor studies, there was a significant impairment of angiogenesis and wound closure in homozygous- and heterozygous-null mice compared with control mice, and indifference between heterozygous- and homozygous-null groups in these measurements (Fig. 5, C and D). These findings further confirmed a gene dosage effect of δ-catenin in angiogenesis. Deletion of one copy of δ-catenin is sufficient to impair pathological angiogenesis, and thus contributes to impaired tumor growth and wound healing in vivo. The experiment was repeated once, and consistent results were observed.
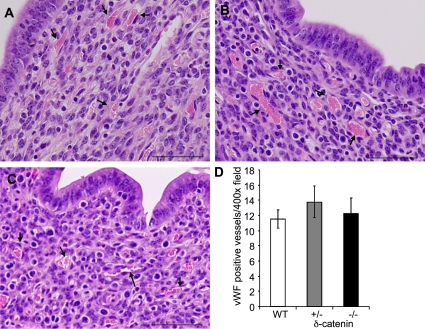
Remarkably, an analysis of the function of δ-catenin in physiological angiogenesis in uterus, as it occurs during the female reproduction cycle, demonstrated no difference in hormonally induced physiological angiogenesis among different groups (Fig. 6). The experiment was repeated once and similar results were observed. This result is consistent with the phenotype that δ-catenin–null mice are fertile and produce a similar number of offspring as the wild-type littermates. Collectively, our data reveal a specific role of δ-catenin in pathological angiogenesis in a gene dosage dependent manner. These notable observations resulting from heterozygous deletion of δ-catenin demonstrate the sensitive nature of pathological angiogenesis to subtle changes of δ-catenin levels.
Figure 6.
Deletion of δ-catenin in mice has no effect on hormone-induced angiogenesis in uterus. Female mice were superovulated, and the uteruses were harvested from wild-type littermates (A), heterozygous-null (B), and homozygous-null δ-catenin (C) mice. Hematoxylin and eosin staining was performed on tissue sections (A–C) for histological analysis. Arrows point to blood vessels. The tissue sections were also probed with a vWF antibody for vascular density. Positive vessels were counted from 10 randomly selected high-power fields under microscopy. Mean and SE were plotted (D). Bar, 200 µm.
Inflammatory cytokines induce the expression of δ-catenin in vascular endothelial cells, and δ-catenin levels are elevated in human lung cancer
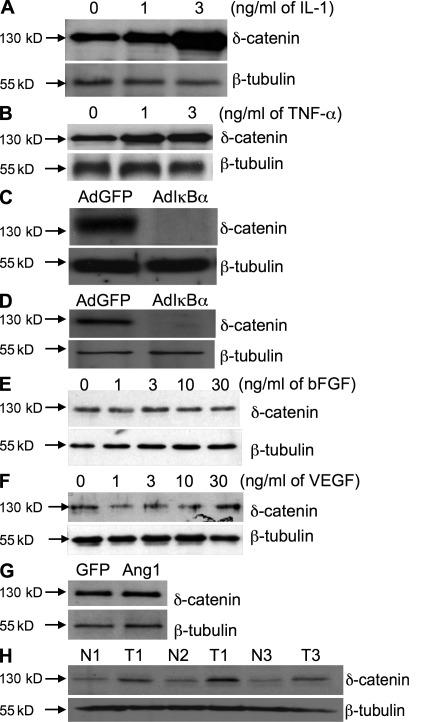
To investigate the molecular mechanisms linking δ-catenin specifically to pathological angiogenesis, we studied gene expression. We found that inflammatory cytokines, including TNF and IL-1, increased δ-catenin expression in a dose-dependent manner in vascular endothelial cells (Fig. 7, A and B), and that gene induction depended on NF-κB, a key regulator of inflammation. Neutralization of NF-κB activation using a mutant IκBα construct completely blocked inflammatory cytokine-induced δ-catenin expression in endothelial cells (Fig. 7, C and D). In contrast, angiogenic factors such as basic fibroblast growth factor, vascular endothelial growth factor, and angiopoietin 1 failed to induce δ-catenin expression (Fig. 7, E–G).
Figure 7.
Inflammatory cytokines induce the expression of δ-catenin through NF-κB in vascular endothelial cells. HUVECs were treated with increasing doses of either IL-1 (A) or TNF (B) for 24 h, and cell lysate was subjected to Western blotting for δ-catenin and β-tubulin expression. HUVECs were infected with adenoviral vectors expressing either GFP or a mutant IκBα for 24 h, followed by stimulation with recombinant IL-1 (C) or TNF (D) at 3 ng/ml for another 24 h. δ-Catenin expression was evaluated by Western blot. HUVECs were treated with increasing doses of either recombinant bFGF (E) or VEGF (F) for 24 h, and cell lysate was subjected to Western blotting for δ-catenin expression. (G) HUVECs were infected with adenoviral vector expressing GFP or Ang1 for 48 h, respectively. δ-Catenin expression was detected by Western blot. (H) Human lung tumor tissues (T) and adjacent normal lung tissues (N) from biopsies were subjected to Western blotting with δ-catenin or β-tubulin antibodies (n = 3 pairs).
A major difference separating physiological angiogenesis in normal growth from pathological angiogenesis in diseases is inflammation. Inflammatory cytokines often possess proangiogenic properties and activate angiogenic genes, as in our observation in δ-catenin in angiogenesis in this study. Because inflammation is elevated in cancer tissues and inflammatory cytokines up-regulate δ-catenin expression, we examined δ-catenin expression in tumor tissues. Consistently, elevated levels of δ-catenin were clearly observed in human lung cancer samples compared with corresponding adjacent normal lung tissues (Fig. 7 H). These findings further support our assessment and link δ-catenin to pathological angiogenesis through inflammation.
DISCUSSION
It is apparent that molecular mechanisms underlying the development of the nervous system have been co-opted by the vasculature. Certain neuronal guidance molecules also regulate angiogenesis and vascular patterning (Mukouyama et al., 2002; Carmeliet and Tessier-Lavigne, 2005; Weinstein, 2005; Jones and Li, 2007). In this study, we demonstrate that a neuronal catenin of the p120 family, δ-catenin, is also expressed in vascular endothelium. δ-Catenin regulates endothelial cell motility and vascular network assembly via modulation of Rho GTPase activity. Most importantly, we observed a gene dosage effect and haploinsufficiency of δ-catenin in pathological angiogenesis. Deletion of one allele of δ-catenin is sufficient to inhibit angiogenesis in tumor progression and wound healing, but has no effect on hormone-induced physiological angiogenesis in the uterus. Collectively, this study identifies a novel function of δ-catenin in vascular biology associated with disease conditions.
This work is the first to document δ-catenin expression in the vascular endothelium. This expression pattern is not surprising, as there have been several proteins that are expressed in both neural and endothelial tissues, and regulate the branching morphogenesis of both systems. These molecules include members of the Ephrin-Eph family, Slit/Robo families, and the Netrins and Semaphorins (Eichmann et al., 2005). δ-Catenin is yet another protein that plays a role in both tissue types. Consistent with the role of δ-catenin in neural cell motility (Lu et al., 1999; Martinez et al., 2003; Kosik et al., 2005), we show here that δ-catenin regulates endothelial cell motility, vascular assembly, and angiogenesis.
Rho family GTPases, including RhoA, Rac, and Cdc42, are key mediators of cytoskeleton dynamics and cell motility. There is strong evidence that p120 and δ-catenin regulate cell motility, at least in part, through modulation of Rho GTPase activation (Kosik et al., 2005; Reynolds, 2007). p120, the prototype member of the family, exhibits opposite function in regulation of Rho GTPase activation. On the one hand, it activates Rac and Cdc42. On the other hand, it inhibits RhoA activation. Consistent with the findings in p120, we found that δ-catenin activates Rac1 and Cdc42, and inhibits RhoA activation in endothelial cells. In searching for GEFs that might interact with δ-catenin in endothelial cells, we found that Vav1, the major isoform of Vav family proteins in endothelial cells, associates with δ-catenin. Overexpression of Vav1 increases Rac1 and Cdc42 activities, whereas knockdown of Vav1 decreases these activities. These data imply that δ-catenin activates Rac1 and Cdc42, likely through recruitment of Vav1 in endothelial cells. Likewise, a study links Vav2 to p120 in Rac and Cdc42 activation in epithelial and fibroblast cells (Noren et al., 2000). Although p120 can clearly act in a GDI-like fashion to directly inhibit RhoA, new evidence suggests that p120 may also inhibit RhoA via recruitment of p190RhoGAP to cadherin (Wildenberg et al., 2006; Reynolds, 2007). It is conceivable to believe a similar mechanism of δ-catenin in suppression of RhoA activation in endothelial cells.
One of the major findings from the study is haploinsufficiency of δ-catenin in endothelial cell motility and angiogenesis. Deletion of one allele of the gene significantly inhibits angiogenesis in tumors and wound healing. This gene dosage effect is well supported by our in vitro data. A reduction of δ-catenin levels results in a significant inhibition of Rho GTPase activity, and impairs endothelial cell motility and vascular structure assembly in vitro. There is no difference in the context of Rho GTPase activity and angiogenic functions between heterozygous- and homozygous-null cells. Importantly, the haploinsufficiency of δ-catenin is also supported by genetic evidence in patients with Cri-du-chat syndrome. These patients often lack one copy of the δ-catenin gene because of a deletion of regions of the short arm of chromosome 5. They show a profound delay in development and neuronal defects, as well as congenital heart defects, including ventricular septal defects in 15–20% of patients (Hills et al., 2006). As endothelial motility is an essential step in cardiovascular development, our findings may provide molecular evidence linking haploinsufficiency of δ-catenin with congenital heart defects associated with Cri-du-chat syndrome. Collectively, our findings reflect the sensitive nature of pathological angiogenesis to subtle changes of δ-catenin levels. Targeting this protein may offer an effective approach for controlling angiogenic diseases.
Another important finding from this work is linking δ-catenin specifically to pathological angiogenesis. Deletion or reduction of the gene inhibited angiogenesis associated with tumor progression and skin wound healing, as both processes are tightly linked to inflammation. In contrast, there is no difference in hormone-induced physiological angiogenesis in the uterus among different groups. Angiogenesis is the body’s response to tissue growth and regeneration, including normal growth during development and growth in disease conditions. One of the major differences between pathological angiogenesis and physiological angiogenesis is inflammation commonly associated with disease conditions. Inflammation, an initial response to tissue injury and insult, often functions as an inducer for pathological angiogenesis. Consistently, we found that inflammatory cytokines, but not angiogenic factors, induce δ-catenin expression. In addition, the levels of δ-catenin are elevated in human lung cancer samples compared with corresponding surrounding normal lung tissues, as inflammation is closely associated with cancer progression. These data may explain the association of δ-catenin specifically to pathological angiogenesis, such as with cancer. Similarly, elevated δ-catenin expression has been detected in some human prostate cancer samples (Lu et al., 2005).
In summary, this study identifies a new function of δ-catenin in vascular endothelium. It is conceivable to believe that tissue injury triggers inflammation, inflammation induces δ-catenin expression in endothelial cells, and that this expression subsequently mediates endothelial cell motility and angiogenesis in diseases in a gene dosage–sensitive manner. This notable observation of haploinsufficiency of δ-catenin in pathological angiogenesis points to a potential utility for targeting the gene in antiangiogenic therapy.
MATERIALS AND METHODS
Mice and cell lines.
δ-Catenin–null mice (Israely et al., 2004) on a C57/Bl6 background were provided by Dr. Xin Liu (University of California, Los Angeles, Los Angeles). δ-Catenin heterozygous-null mice were bred to generate wild-type littermates, heterozygous-null, and homozygous-null mice. All mice used in this work were housed in pathogen-free units at Vanderbilt University Medical Center. Animal protocols were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Age- and sex-matched mice were used in each study.
HUVECs were purchased from Lonza. Endothelial cells were grown on 0.1% gelatin-coated tissue culture plates in endothelial growth medium for microvascular cells type two (EGM-2; Lonza). Cells between passages 3–7 were used. Lewis lung adenocarcinoma cells were grown on tissue culture plates in DME supplemented with 10% FBS and 1% antibiotics. Human neuronal cell lines (U251MG and HT-22), human embryonic kidney epithelial cells (293), and human leukocyte cell lines (THP-1 and U937) were grown in DME or RPMI1640 with 10% FBS. All cells were maintained in a humidified incubator with 5% CO2 at 37°C. Murine lung endothelial cells were isolated and evaluated as previously described (Kamiyama et al., 2006). In brief, lungs were harvested from age- and sex-matched wild-type littermates, C/EBP-δ heterozygous, and C/EBP-δ–null mice, minced, and digested with 0.25% Trypsin-EDTA. The single-cell suspension was cultured in EGM (Clonetics) for 5–6 d, followed by incubation with FITC-conjugated anti-mouse CD31 (BD). CD31-positive cells were sorted with a FACStarPlus flow cytometer (BD). Cells used in each study were >95% pure.
In vitro angiogenic assays.
Angiogenic assays were performed as previously described (Kamiyama et al., 2006). Endothelial cell migration was performed in Transwell assays using 25 ng/ml VEGF (R&D Systems) as a chemoattractant. Vascular tubule formation was carried out in growth factor reduced Matrigel (BD). Cell proliferation was measured by BrDU incorporation and analyzed on a FACScan flow cytometer (BD).
In vivo angiogenesis models.
Lewis lung adenocarcinoma cell tumor cells (5 × 105 per mouse) were injected subcutaneously into the left hind flank of 6-wk-old female δ-catenin homozygous-null, δ-catenin heterozygous-null, or wild-type mice. Tumor growth was measured daily by caliper. Tumor volume was calculated as length × width2/2. At the end of the experiment, tumor tissues were harvested, freshly frozen, and embedded in OCT. 7-µm sections were cut and subjected to immunohistochemistry. The tumor study was repeated twice with seven mice per group.
Two wounds were made on the back of each mouse using a 6-mm skin biopsy punch. The wound dimensions were measured daily and the wound area was calculated as π(long radius)(short radius). The percent change was calculated as (initial wound area) − (current wound area)/(initial wound area). The experiment was repeated once with seven mice per group.
4-5-wk-old female mice were treated with gonadotropin from pregnant mare serum (Sigma-Aldrich) for 48 h, followed by human chorionic gonadotropin (Sigma-Aldrich) for 13–15 h. Uteruses were then harvested and processed for immunohistochemistry. The experiment was repeated once with six mice per group.
Immunofluorescent staining.
Monolayer of HUVECs was fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and incubated with a δ-catenin–specific antibody (BD) and Cy2-conjugated secondary antibody. Tumor tissue sections were incubated with antibodies against δ-catenin or CD31 (BD), followed by incubation with fluorescently conjugated secondary antibody. Tissues were harvested from δ-catenin–null mice were used as a negative control.
Western blot and immunoprecipitation.
Cellular lysates were analyzed by Western blot and incubated with a specific antibody against δ-catenin (Santa Cruz Biotechnology, Inc.). For immunoprecipitation, cell lysates were preincubated with Protein A/G agarose (Santa Cruz Biotechnology, Inc.). The samples were then centrifuged, and supernatants were used for coimmunoprecipitation experiments. Immunoprecipitations were performed with antibodies against either Vav1 (Santa Cruz Biotechnology, Inc.) or δ-catenin (Santa Cruz Biotechnology, Inc.) overnight, followed by incubation with Protein A/G agarose. Agarose beads were washed in cell lysis buffer and boiled with gel loading buffer. Samples were then subjected to SDS-PAGE and Western blot analysis.
Overexpression and knockdown.
For overexpression, cells were electroporated with plasmids using a Nucleofector device (Lonza). Knockdown of expression was performed by shRNA panels (Sigma-Aldrich) and transfected the constructs into endothelial cells by Amaxa.
Rac, RhoA, and Cdc42 activation assay.
Rac1, RhoA, and Cdc42 activation kits were purchased from Chemicon. The assays were performed following manufacture’s instructions.
δ-Catenin induction in endothelial cells.
HUVECs were stimulated with recombinant TNF, IL-1β (R and D), basic fibroblast growth factor, and vascular endothelial growth factor for 24 h, followed by determination of δ-catenin levels by Western blot using specific antibody. The adenoviral vectors directing the expression of Ang1 (AdAng1) and a mutated IκBα that can’t be phosphorylated as a NF-κB inhibitor (AdIκB), and control GFP (AdGFP) were described previously (Kobayashi et al., 2006; Kamiyama et al., 2006). HUVECs were infected with the viral vectors at a multiplicity of infection of 10.
Statistics.
Results are reported as mean + SE for each group. Parametric, Student’s t test and nonparametric, Mann-Whitney U test, were used to analyze statistical differences between control and treated groups. Tests of hypothesis between groups were made with analysis of variance with adjusted least square means. Statistical calculations were run on Prism 5 Graph Pad. Differences were considered statistically significant if P < 0.05.
Online supplemental material.
Fig. S1 shows murine 3LL tumor tissue sections harvested from wild-type and δ-catenin–null mice. Fig. S2 shows that knockdown of δ-catenin impairs angiogenic phenotype, whereas δ-catenin overexpression enhances an angiogenic phenotype in vitro. Fig. S3 shows that modulation of δ-catenin levels affect Rho GTPase activity. Fig. S4 shows that tumor vascular density is reduced in heterozygous and homozygous δ-catenin–null mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091097/DC1.
Acknowledgments
We thank Drs. Vivian Siegel and Jin Chen at Vanderbilt University Medical Center for critical comments and editing on the manuscript.
This work is supported in part by grants from National Institutes of Health (NIH; CA108856, NS45888, and AR053718) to P.C. Lin and training grants from NIH to L.M. DeBusk (T32CA009592) and K.C. Boelte (T32CA009582).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- HUVEC
- human umbilical vein endothelial cell
- shRNA
- short hairpin RNA
References
- Bustelo X.R. 2000. Regulatory and signaling properties of the Vav family. Mol. Cell. Biol. 20:1461–1477 doi: 10.1128/MCB.20.5.1461-1477.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Tessier-Lavigne M. 2005. Common mechanisms of nerve and blood vessel wiring. Nature. 436:193–200 doi: 10.1038/nature03875 [DOI] [PubMed] [Google Scholar]
- Eichmann A., Makinen T., Alitalo K. 2005. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 19:1013–1021 doi: 10.1101/gad.1305405 [DOI] [PubMed] [Google Scholar]
- Georgiades P., Ogilvy S., Duval H., Licence D.R., Charnock-Jones D.S., Smith S.K., Print C.G. 2002. VavCre transgenic mice: a tool for mutagenesis in hematopoietic and endothelial lineages. Genesis. 34:251–256 doi: 10.1002/gene.10161 [DOI] [PubMed] [Google Scholar]
- Grosheva I., Shtutman M., Elbaum M., Bershadsky A.D. 2001. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114:695–707 [DOI] [PubMed] [Google Scholar]
- Hatzfeld M. 2005. The p120 family of cell adhesion molecules. Eur. J. Cell Biol. 84:205–214 doi: 10.1016/j.ejcb.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Hills C., Moller J.H., Finkelstein M., Lohr J., Schimmenti L. 2006. Cri du chat syndrome and congenital heart disease: a review of previously reported cases and presentation of an additional 21 cases from the Pediatric Cardiac Care Consortium. Pediatrics. 117:e924–e927 doi: 10.1542/peds.2005-1012 [DOI] [PubMed] [Google Scholar]
- Israely I., Costa R.M., Xie C.W., Silva A.J., Kosik K.S., Liu X. 2004. Deletion of the neuron-specific protein delta-catenin leads to severe cognitive and synaptic dysfunction. Curr. Biol. 14:1657–1663 doi: 10.1016/j.cub.2004.08.065 [DOI] [PubMed] [Google Scholar]
- Jin K., Zhu Y., Sun Y., Mao X.O., Xie L., Greenberg D.A. 2002. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 99:11946–11950 doi: 10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.A., Li D.Y. 2007. Common cues regulate neural and vascular patterning. Curr. Opin. Genet. Dev. 17:332–336 doi: 10.1016/j.gde.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama M., Pozzi A., Yang L., DeBusk L.M., Breyer R.M., Lin P.C. 2006. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 25:7019–7028 doi: 10.1038/sj.onc.1209694 [DOI] [PubMed] [Google Scholar]
- Kobayashi H., DeBusk L.M., Babichev Y.O., Dumont D.J., Lin P.C. 2006. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 108:1260–1266 doi: 10.1182/blood-2005-09-012807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K.S., Donahue C.P., Israely I., Liu X., Ochiishi T. 2005. Delta-catenin at the synaptic-adherens junction. Trends Cell Biol. 15:172–178 doi: 10.1016/j.tcb.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Lee P., Goishi K., Davidson A.J., Mannix R., Zon L., Klagsbrun M. 2002. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc. Natl. Acad. Sci. USA. 99:10470–10475 doi: 10.1073/pnas.162366299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Paredes M., Medina M., Zhou J., Cavallo R., Peifer M., Orecchio L., Kosik K.S. 1999. δ-Catenin, an adhesive junction-associated protein which promotes cell scattering. J. Cell Biol. 144:519–532 doi: 10.1083/jcb.144.3.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Dobbs L.J., Gregory C.W., Lanford G.W., Revelo M.P., Shappell S., Chen Y.H. 2005. Increased expression of delta-catenin/neural plakophilin-related armadillo protein is associated with the down-regulation and redistribution of E-cadherin and p120ctn and human prostate cancer. Hum. Pathol. 36:1037–1048 [DOI] [PubMed] [Google Scholar]
- Martinez M.C., Ochiishi T., Majewski M., Kosik K.S. 2003. Dual regulation of neuronal morphogenesis by a delta-catenin-cortactin complex and Rho. J. Cell Biol. 162:99–111 doi: 10.1083/jcb.200211025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukouyama Y.S., Shin D., Britsch S., Taniguchi M., Anderson D.J. 2002. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 109:693–705 doi: 10.1016/S0092-8674(02)00757-2 [DOI] [PubMed] [Google Scholar]
- Noren N.K., Liu B.P., Burridge K., Kreft B. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150:567–580 doi: 10.1083/jcb.150.3.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuyse B., Moons L., Storkebaum E., Beck H., Nuyens D., Brusselmans K., Van Dorpe J., Hellings P., Gorselink M., Heymans S., et al. 2001. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 28:131–138 doi: 10.1038/88842 [DOI] [PubMed] [Google Scholar]
- Reynolds A.B. 2007. p120-catenin: Past and present. Biochim. Biophys. Acta. 1773:2–7 doi: 10.1016/j.bbamcr.2006.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V.L., Ardouin L., Prisco A., Reynolds L.F. 2003. Vav1: a key signal transducer downstream of the TCR. Immunol. Rev. 192:42–52 doi: 10.1034/j.1600-065X.2003.00032.x [DOI] [PubMed] [Google Scholar]
- Weinstein B.M. 2005. Vessels and nerves: marching to the same tune. Cell. 120:299–302 doi: 10.1016/j.cell.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Wildenberg G.A., Dohn M.R., Carnahan R.H., Davis M.A., Lobdell N.A., Settleman J., Reynolds A.B. 2006. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 127:1027–1039 doi: 10.1016/j.cell.2006.09.046 [DOI] [PubMed] [Google Scholar]