Abstract
Inflammatory lymphangiogenesis plays a crucial role in the development of inflammation and transplant rejection. The mechanisms of inflammatory lymphangiogenesis during bacterial infection, toll-like receptor ligand administration, and wound healing are well characterized and depend on ligands for the vascular endothelial grow factor receptor (VEGFR) 3 that are produced by infiltrating macrophages. But inflammatory lymphangiogenesis in nonlymphoid tissues during chronic viral infection is unstudied. Herpes simplex virus 1 (HSV-1) infection of the cornea is a leading cause of blindness and depends on aberrant host immune responses to antigen within the normally immunologically privileged cornea. We report that corneal HSV-1 infection drives lymphangiogenesis and that corneal lymphatics persist past the resolution of infection. The mechanism of HSV-1–induced lymphangiogenesis was distinct from the described mechanisms of inflammatory lymphangiogenesis. HSV-1–elicited lymphangiogenesis was strictly dependent on VEGF-A/VEGFR-2 signaling but not on VEGFR-3 ligands. Macrophages played no role in the induction of lymphangiogenesis and were not a detectable source of VEGF-A. Rather, using VEGF-A reporter transgenic mice, we have identified infected epithelial cells as the primary source of VEGF-A during HSV-1 infection. Our results indicate that HSV-1 directly induces vascularization of the cornea through up-regulation of VEGF-A expression.
Although the regulation of developmental and inflammatory angiogenesis is well characterized, the study of lymphangiogenesis is still in its infancy despite the multitude of critical roles performed by the lymphatic vasculature. The primary function of the lymphatic vascular system is to drain fluid from the extracellular matrix (Oliver, 2004; Cueni and Detmar, 2008). In addition to maintaining low tissue pressure, this action also serves to deliver antigen and cytokines from sites of inflammation to draining lymph nodes (Oliver, 2004; Cueni and Detmar, 2008). Lymphatic capillaries are the primary route for the transport of antigen-loaded APCs to draining lymph nodes (von Andrian and Mempel, 2003), and tissues devoid of lymphatic vasculature, such as the cornea and brain, are characterized by immune privilege (Streilein, 2003; Cursiefen, 2007; Galea et al., 2007; Lambe et al., 2007). Lymphatic drainage is crucial to the development of immunity but, conversely, can contribute to destructive inflammation during graft rejection, autoimmunity, and chronic infection (Paavonen et al., 2002; Kerjaschki et al., 2004; Baluk et al., 2005; Fricke et al., 2007).
As a result, considerable investment has been made to clarify the mechanisms of inflammatory lymphangiogenesis, the growth of lymphatic vessels from preexisting vasculature. Studies of chronic inflammation during graft rejection, bacterial infection, and after administration of inflammatory cytokines, such as fibroblast growth factor b and IL-1β, have suggested common lymphangiogenic mechanisms between these models. The initiation of lymphangiogenesis is characterized by the recruitment of activated macrophages to a site of inflammation and subsequent production of the prolymphangiogenic cytokines vascular endothelial growth factor (VEGF) C or D (Cursiefen et al., 2003, 2004b; Watari et al., 2008; Kataru et al., 2009). Ligation of the lymphatic vessel endothelial cell expressed receptor, VEGF receptor (VEGFR) 3, by these cytokines induces mitosis and lymphatic vessel branching toward their source (Podgrabinska et al., 2002; Hong et al., 2004b; Oliver, 2004). Blockade of this pathway, either through inhibition of VEGF-C/D/VEGFR-3 signaling or depletion of macrophages, profoundly inhibits inflammatory lymphangiogenesis (Cursiefen et al., 2004b; Cueni and Detmar, 2008; Kataru et al., 2009). In vitro and overexpression studies also indicate that the related cytokine VEGF-A is capable of directly inducing lymphangiogenesis through ligation of the receptor VEGFR-2 (Cueni and Detmar, 2008). However, in vivo the lymphangiogenic effects of VEGF-A are believed to be secondary to the more potent prolymphangiogenic cytokines VEGF-C and -D during inflammatory lymphangiogenesis outside of nonlymphoid tissues (Cursiefen et al., 2004b; Cueni and Detmar, 2008). However, no studies focused on lymphangiogenesis during chronic viral infection have been published and the extent to which known mechanisms of inflammatory lymphangiogenesis apply during this context is unknown.
Herpes simplex virus 1 (HSV-1) is among the most prevalent human infections with worldwide seroprevalence rates ranging from 50 to 90% (Smith and Robinson, 2002). Infection is lifelong and characterized by frequent reactivation of latent virus within the trigeminal nerve. Newly created virions travel down the sensory fibers of the trigeminal nerve, usually resulting in the development of orolabial lesions. However, the trigeminal nerve also provides sensory fibers to the cornea. Thus, reactivation from latency occasionally results in the transport of virions to the cornea, resulting in recurring bouts of inflammatory keratitis. Herpes simplex keratitis (HSK) is among the most severe consequences of HSV-1 infection and is believed to be the leading cause of corneal blindness in the developed world (Liesegang et al., 1989) as a result of blinding corneal opacity elicited by the episodic inflammatory response (Carr et al., 2001; Wickham and Carr, 2004; Kaye and Choudhary, 2006). The potentially blinding complications associated with HSK require viral replication within the cornea. However, HSK is believed to be a product of chronic inflammation mediated by the host immune response because CD4+ T cells are required for the induction of HSK, as are intact lymph nodes draining the sclera and cornea (Carr et al., 2001; Wickham and Carr, 2004; Biswas et al., 2006).
The extension of blood vessels from surrounding tissues into the cornea is a common complication during HSK and contributes to inflammation as well as direct impairment of vision (Carr et al., 2001; Kaye and Choudhary, 2006; Biswas et al., 2006). However, our group has found that in addition to inducing corneal hemangiogenesis, ocular HSV-1 infection induces the extension of lymphatic vessels into the cornea. In sharp contrast to inflammatory lymphangiogenesis during chronic bacterial infections, we report that HSV-1–induced lymphangiogenesis is not dependent on macrophage recruitment or on the expression of the cytokines VEGF-C or D. Rather, lymphangiogenesis was exquisitely dependent on up-regulated expression of VEGF-A, a cytokine which is typically associated with angiogenesis. Intriguingly, the source of VEGF-A during corneal HSV-1 infection was not an infiltrating leukocyte population but was instead determined to be HSV-1–infected corneal epithelial cells. Our results indicate that HSV-1 directly influences the vascular environment of the cornea through VEGF-A expression. HSV-1 induces inflammatory lymphangiogenesis in the cornea by a mechanism distinct from previously described models of inflammatory lymphangiogenesis and is not simply an innate response to viral infection, as infection with vesicular stomatitis virus (VSV) did not up-regulate expression of VEGF-A in infected cells.
RESULTS
HSV-1 infection induces corneal lymphangiogenesis
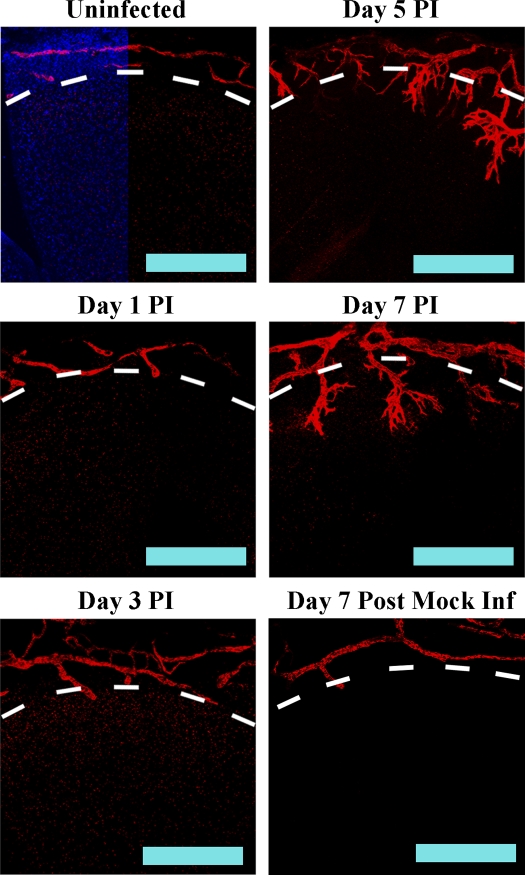
Although the normal cornea is devoid of blood and lymphatic vessels, it is surrounded by rings of both vessel types in the region proximal to the cornea, the limbus. To determine if HSV-1 infection induced sprouting of limbal lymphatics into the cornea, C57BL/6 mice were infected with 105 PFU HSV-1 and corneas were harvested at 1, 3, 5, and 7 d post infection (PI). Confocal microscopic examination of corneas for the lymphatic vessel-specific antigen LYVE-1 indicated that HSV-1 induced rapid sprouting of limbal lymphatics. Small sprouts were observed as early as day 1 PI (Fig. 1). Sprouts continued extension into the cornea through day 7 PI, which was the last time point analyzed using 105 PFU HSV-1 because of animal mortality. In contrast, mock-infected animals, which were scarified but received PBS rather than an inoculum of HSV-1, did not show infiltration of lymphatic vessels into the cornea (Fig. 1, bottom right). Thus, HSV-1 infection elicited corneal lymphangiogenesis.
Figure 1.
HSV-1 infection induces lymphangiogenesis. WT mice were scarified and then either saline (mock) or 105 PFU HSV-1 was applied to the cornea. The corneas were harvested at the indicated day PI and stained for the lymphatic vessel antigen LYVE-1 (red). In an uninfected mouse cornea, lymphatic vessels are found in the limbus but do not extend into the cornea (marked by dashed line). After HSV-1 infection, limbal lymphatics sprouts and vessels extend into the cornea proper. Images are representative of a minimum of three independent experiments with a total of n = 12 corneas per time point. Bars, 100 µm.
Inflammatory lymphangiogenesis during HSV-1 infection is comparable to angiogenesis, and corneal lymphatics exist beyond resolution of infection
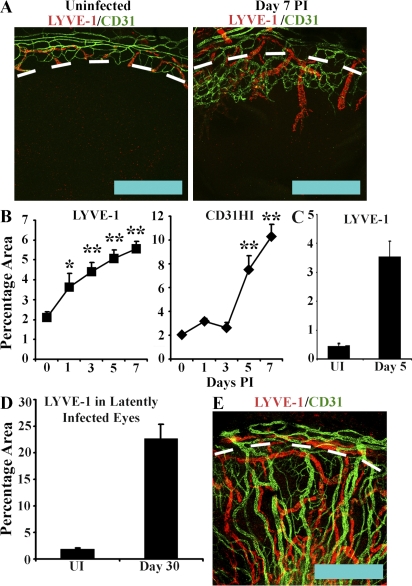
Angiogenesis is a well recognized complication of HSK that is believed to contribute to both corneal opacity and the increased risk of corneal transplant rejection observed in HSK-affected patients. However, corneal lymphangiogenesis is believed to be at least as significant in graft rejection as angiogenesis. As HSV-1–induced lymphangiogenesis has previously not been described, we sought to compare the relative rates of blood and lymphatic vessel extension into the cornea after corneal HSV-1 infection. Blood vessels exhibit high expression of CD31 with no LYVE-1 expression, whereas lymphatic vessels express high levels of LYVE-1 and low or no CD31. Corneas were harvested at 1, 3, 5, and 7 d PI from C57BL/6 mice and examined for expression of LYVE-1 and CD31. Harvested corneas included both limbal and corneal tissue as the delineation between corneal and limbal tissue. In addition, the preexisting ring of limbal lymphatic vessels provided convenient and consistent reference points for ensuring equivalent orientation of scans between different corneas. By day 7 PI, blood and lymphatic vessels showed comparable extension into the cornea (Fig. 2 A). However, the kinetics of growth between the two vessel types differed. Quantitative analysis of percentage areas positive for LYVE-1 and CD31HI expression in random 100× fields indicated that lymphangiogenesis preceded angiogenesis. Lymphangiogenesis was observed as early as day 1 PI with rapid increases in LYVE-1+ percentage areas through day 5 PI (Fig. 2 B). In contrast, angiogenesis was delayed, with no detectable increase in CD31HI percentage area until day 5 PI, after which angiogenesis appeared to occur more rapidly than lymphangiogenesis (Fig. 2 B).
Figure 2.
HSV-1–induced lymphangiogenesis is comparable to hemangiogenesis and persists beyond resolution of infection. To compare the relative rates of lymphangiogenesis and hemangiogenesis, C57BL/6 mice were infected with 105 PFU HSV-1 and examined for LYVE-1 (red) and CD31 (green) expression. (A) In uninfected animals, blood (CD31HI) and lymphatic vessels (LYVE-1+) are confined to the limbus (dashed line), but by day 7 PI, both vessel types extend into the cornea. Extension of lymphatic vessels is comparable to that of blood vessels. Bars, 500 µm. This panel is representative of two experiments with a total of eight corneas. (B) The kinetics of lymphangiogenesis and hemangiogenesis differ with infiltration of LYVE-1+ vessels preceding that of CD31HI blood vessels, as shown by semiquantitative analysis of LYVE-1+ and CD31HI expression indicated as percent area of 100× fields of view as a function of days PI with 105 PFU HSV-1. The results depicted are a summary of two experiments with eight corneas per time point and four independent 100× fields of view quantified per cornea. The results are displayed as mean ± SEM percentage area. *, P < 0.05; **, P < 0.01, comparing infected to uninfected (UI). (C) To determine if dilation of limbal lymphatics could be contributing to increases in LYVE-1+ percent area measurements, corneas were harvested from animals that were either uninfected or at day 5 PI and quantified for LYVE-1 expression. LYVE-1+ area at day 5 PI was significantly elevated (**, P < 0.01) compared with the uninfected. The increase in percent area was comparable to that observed in corneas harvested with limbal lymphatics (B, day 0 to day 5 PI LYVE-1+ area percentage increase of 2.99 ± 0.7% vs. a LYVE-1+ area percentage increase of 3.3 ± 0.6%), indicating that LYVE-1+ percent increase was not simply the result of limbal lymphatic dilation. (D) To determine if HSV-1–induced lymphatics existed in corneas past the resolution of viral replication, C57BL/6 mice were infected with 103 PFU HSV-1 and followed to day 30 PI. LYVE-1+ percentage area was significantly (P < 0.01) elevated relative to uninfected controls. Error bars in C and D represent the SEM based on the results of each cornea sample summarized for both experiments. (E) Both LYVE-1+ lymphatic and CD31HI blood vessels were observed throughout the corneas of latently infected animals. Confocal images of representative corneas from animals latently infected with 103 PFU HSV-1 showing LYVE-1+ and CD31+ vessels at day 30 PI. Bar, 500 µm. Graphs shown in C and D are a summary of two experiments with four corneas per group per time point of four independent 100× fields of view quantified per cornea.
As quantified scans included both limbal and corneal tissue, the increases in total LYVE-1+ and CD31+ areas could not be simply the result of remodeling of existing limbal lymphatics into the cornea proper, as total LYVE-1+ and CD31+ area would be unchanged in this case. Examination of corneas harvested without limbal tissue showed an ∼3.28 ± 0.64% increase in LYVE-1+ percentage area between uninfected versus corneas harvested at day 5 PI (Fig. 2 C, uninfected 0.45 ± 0.07% vs. day 5 PI 3.73 ± 0.57% LYVE-1+ area). This increase was not significantly different from that observed between uninfected corneas versus corneas harvested at day 5 PI with limbal tissue containing preexisting limbal lymphatics (Fig. 2 B, increase in percentage of LYVE-1+ area from day 0 to day 5 was 2.99 ± 0.66%). As far as could be determined within the limitations of our experimental design, the bulk of the increase in corneal LYVE-1+ area after HSV-1 occurred in the cornea proper. Thus, the increase in LYVE-1+ area was unlikely to be the result of either limbal lymphatic vessel dilation or remodeling of limbal lymphatics into the cornea.
HSV-1 disease in humans is characterized by recurring cycles of active viral replication followed by latency. During latent infection, virus is not detectable in the cornea and inflammatory cytokine levels are comparable to those observed in normal corneas (He et al., 1999; Carr et al., 2009). Although mature blood and lymphatic vessels do not require continuous cytokine stimulation for maintenance, immature vessels undergo destruction after withdrawal of angiogenic or lymphangiogenic stimuli (Jain, 2003; Karpanen and Alitalo, 2008). Corneas were harvested from animals latently infected with 103 PFU HSV-1 30 d earlier. A lower inoculum of 103 PFU HSV-1 rather than 105 PFU was used for this portion of the study because of excessive animal mortality using the higher dose. By day 30 PI, corneal LYVE-1+ percentage area had increased from 1.85 ± 0.22% in uninfected corneas to 22.6 ± 2.75% (Fig. 2 D). As with blood vessels, lymphatic vessels remained after resolution of viral replication (Fig. 2 E), which typically occurs between days 7 and 14 PI depending on inoculum and viral strain. LYVE-1+ vessels exhibited comparable extension past the limbus into the cornea as CD31HI blood vessels. Because of exceedingly high animal mortality, it is unclear whether active lymphangiogenesis is still occurring by day 30 PI or whether the presence of corneal blood and lymphatic vessels is the result of persistence past resolution of angiogenic or lymphangiogenic stimulation. However, it is clear that HSV-1–induced corneal lymphatics remained after resolution of detectable viral replication. Furthermore, HSV-1–induced lymphatic vessels were capable of draining soluble fluorescently labeled dextran from the cornea to the draining lymph nodes and this capability remained at day 30 PI, indicating functionality (Fig. S2). This is in contrast to uninfected eyes in which the placement of soluble fluorescently labeled dextran was not apparent in the draining lymph node after placement in the cornea (Fig. S2).
HSV-1–induced lymphangiogenesis is dependent on VEGF-A/VEGFR-2 but not VEGF-C/D/VEGFR-3
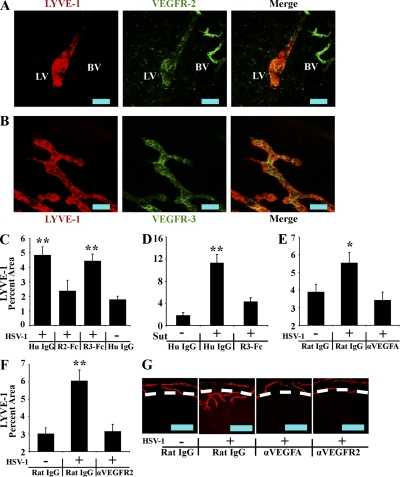
Corneal HSV-1–induced lymphatics express the VEGF family receptors VEGFR-2 and VEGFR-3 (Fig. 3, A and B; isotypic controls shown in Fig. S1), which bind the prolymphangiogenic cytokines VEGF-A/C/D or VEGF-C/D, respectively. To test dependence on these ligands, we administered competitive inhibitors composed of the extracellular domains of either VEGFR-2 or VEGFR-3 fused to the human IgG1 Fc domain, labeled VEGFR2-Fc or VEGFR3-Fc, respectively. Inhibitors were administered by subconjunctival injection to mock- or HSV-1–infected animals. The presence of lymphatic vessels may have an impact on the adaptive immune response to HSV-1, which could theoretically complicate data analysis. Thus, corneas were harvested for examination of LYVE-1 expression at day 5 rather than day 7 PI. Previously described models of inflammatory lymphangiogenesis are inhibited by blockade of VEGFR-3 ligands (Cursiefen et al., 2003, 2004b; Watari et al., 2008; Kataru et al., 2009). However, no inhibition of HSV-1–induced lymphangiogenesis was observed after administration of VEGFR3-Fc (Fig. 3 C), even though VEGFR3-Fc significantly inhibited corneal lymphangiogenesis in response to placement of a full penetration single suture within the cornea (Fig. 3 D). Rather, HSV-1–induced lymphangiogenesis was completely blocked by VEGFR2-Fc (Fig. 3 C), with LYVE-1+ corneal percentage areas being reduced from 4.8% ± 0.6% in control IgG-treated HSV-1–infected corneas to 2.4% ± 0.7% in VEGFR2-Fc–treated HSV-1–infected corneas. The LYVE-1+ percentage area in VEGFR2-Fc–treated corneas was not significantly different from that of control IgG-treated uninfected eyes (1.79 ± 0.2%; Fig. 3 C). Thus, the mechanism of HSV-1–induced lymphangiogenesis was unique in its dependence on VEGFR-2 but not VEGFR-3 ligands.
Figure 3.
HSV-1–induced lymphangiogenesis is dependent on VEGF-A/VEGFR-2 but not VEGFR-3 ligands. (A and B) LYVE-1+ (red) vessels extending into the cornea after HSV-1 infection were positive for VEGFR-2 (A) and VEGFR-3 (B). Isotypic control staining is shown in Fig. S1. LV, lymphatic vessel; BV, blood vessel. Data are representative of two experiments. n = 6 per group. The image was acquired with a 400× objective. Bars, 100 µm. (C) Mock- or HSV-1–infected animals were treated with competitive inhibitors of VEGF family members, including VEGFR2-Fc or VEGFR3-Fc, which bind VEGF-A/C/D or VEGF-C/D, respectively. Lymphatic vessel area was quantified using LYVE-1 and expressed as percent area of 100× fields of view. The inhibitor VEGFR2-Fc significantly (**, P < 0.01) inhibited HSV-1–associated lymphangiogenesis, whereas VEGFR3-Fc was ineffective. (D) VEGFR3-Fc was capable of inhibiting (**, P < 0.01) lymphangiogenesis through 5 d after the placement of a full penetration suture through the cornea when used with the same doses and schedule as with the experiment shown in C. (E and F) To determine if VEGF-A or VEGFR-2 were directly responsible for HSV-1–induced lymphangiogenesis, mock- or HSV-1–infected animals were treated with either control IgG or neutralizing antibody against VEGF-A (E) or VEGFR-2 (F), both of which significantly (**, P < 0.01; *, P < 0.05) inhibited HSV-1–induced lymphangiogenesis. Error bars in C–F represent the SEM based on the results of each cornea sample summarized for both experiments. (G) Representative images of HSV-1–associated lymphatic vessels after subconjunctival injections of control rat IgG, anti–VEGF-A, or anti–VEGFR-2, visualized using LYVE-1 (red). Dashed lines show demarcation between the limbus and the cornea proper. Bars, 500 µm. Data showing lymphatic vessel area after antibody or VEGFR2/3-Fc treatment is a summary of two experiments with 12 corneas and four 100× fields of view quantified per cornea.
The effective inhibitor of HSV-1–induced lymphangiogenesis, VEGFR2-Fc, binds VEGF-A/C/D, whereas VEGFR3-Fc binds VEGF-C/D only, implicating VEGF-A. To test dependence on VEGF-A and its lymphatic vessel–expressed receptor VEGFR-2, monoclonal antibodies against VEGF-A and VEGFR-2 were administered. As with VEGFR2-Fc, anti–VEGF-A and anti–VEGFR-2 completely blocked HSV-1–induced increases in LYVE-1+ percentage areas when compared with animals treated with control IgG, which were either mock infected or infected with HSV-1 (Fig. 3, E and F). Treatment with anti–VEGF-A and anti–VEGFR-2 reduced vessel extension into the cornea (Fig. 3 G), and preexisting limbal lymphatics in treated groups largely resembled those observed in uninfected animals. Thus, the mechanism of HSV-1–induced lymphangiogenesis differs from previously described models in that it is not dependent on VEGFR-3 ligands but VEGF-A/R-2 signaling is required.
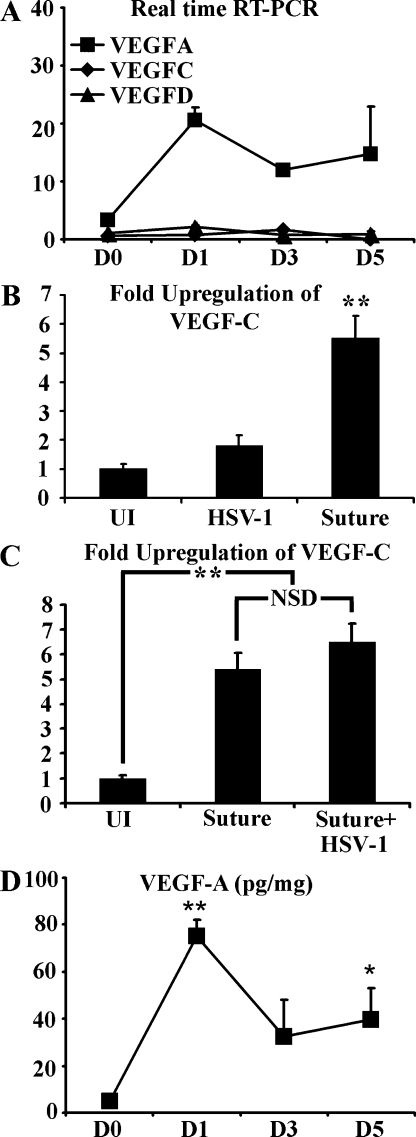
Real-time RT-PCR was used to analyze the levels of transcripts for VEGF-A, C, and D relative to β-actin. The VEGF-A transcript was rapidly up-regulated after HSV-1 infection, with detection as early as day 1 PI and continued expression through day 5 PI (Fig. 4 A). In contrast, no up-regulation of either VEGF-C or D transcript was observed through day 5 PI (Fig. 4 A). Also in contrast, corneal suture placement induced significant up-regulation of VEGF-C by real-time RT-PCR (Fig. 4 B). Notably, HSV-1 infection of sutured corneas did not result in either down-regulation or additional up-regulation of VEGF-C (Fig. 4 C). Protein levels of VEGF-A in the cornea largely mirrored that of VEGF-A transcript with a rapid increase in VEGF-A levels by day 1 (Fig. 4 D). By day 3 PI, VEGF-A levels fell to 32 ± 15 pg/mg but remained elevated above baseline (4.8 ± 0.4 pg/mg of cornea) through day 5 PI (Fig. 4 D). Collectively, our results suggest that acute HSV-1–induced lymphangiogenesis is strictly dependent on VEGF-A/VEGFR-2, whereas VEGFR-3 ligands do not play a role, likely because of the absence of expression during HSV-1 infection.
Figure 4.
VEGF-A expression is up-regulated after HSV-1 infection. (A) Corneal levels of VEGF-A/C/D were quantified by real-time RT-PCR and normalized per 1,000 transcripts of the housekeeping gene β-actin with values given at the indicated day PI. Although HSV-1 infection induced up-regulation of VEGF-A, no up-regulation of VEGF-C or -D was observed. **, P < 0.01, comparing infected to uninfected groups. The figure is a summary of two experiments. n = 3 per group per experiment. (B) However, at day 5 after corneal suture placement VEGF-C messenger RNA was significantly up-regulated relative to both untreated and HSV-1–infected corneas, here expressed as fold induction using the housekeeping gene β-actin for normalization. **, P < 0.01 comparing the suture-induced VEGF-C level to the HSV-1 and uninfected (UI) groups. The figure is a summary of two experiments, n = 3 per group per experiment. (C) To determine if HSV-1 infection may suppress VEGF-C expression, sutured mice were either mock infected or infected with HSV-1 at the time of suture placement and the corneas were collected at day 5 after suture/PI. HSV-1 infection did not affect VEGF-C expression after suture placement. A summary of two experiments is shown with a total of n = 6 per group. **, P<.01 comparing the uninfected to the other two groups. NSD, no significant difference. (D) Protein levels for VEGF-A were measured by cytokine bead array after HSV-1 infection, expressed as picograms of VEGF-A per milligram of wet cornea mass. VEGF-A protein expression was rapidly up-regulated and remained up-regulated throughout the course of the study. **, P < 0.01; *, P < 0.05, comparing levels of VEGF-A protein at time points PI to uninfected (day 0 [D0]) control. The figure is a summary of two experiments, n = 6 corneas per time point. Error bars in A–D represent the SEM based on the results of each cornea sample summarized for both experiments.
Macrophages do not play a role in HSV-1–induced lymphangiogenesis
Previously described models of inflammatory lymphangiogenesis are dependent on macrophage recruitment and activation (Cursiefen et al., 2003, 2004b; Watari et al., 2008; Kataru et al., 2009). Activated macrophages exert prolymphangiogenic effects by two distinct mechanisms. First, activated macrophages express the prolymphangiogenic cytokines VEGF-A/C/D (Cursiefen et al., 2003, 2004b; Watari et al., 2008; Kataru et al., 2009). Second, macrophages and/or other bone marrow–derived populations are capable of transdifferentiation to an endothelial cell phenotype, thereby making a direct structural contribution to the lymphatic vessel wall (Maruyama et al., 2005).
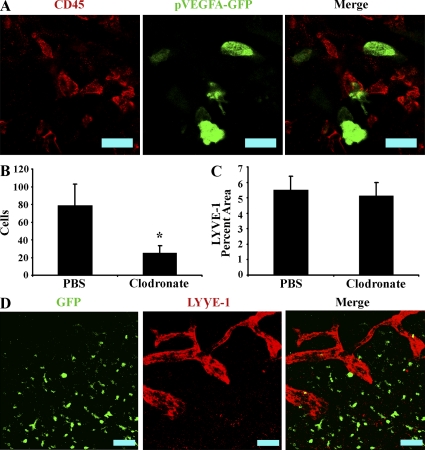
As HSV-1–induced lymphangiogenesis is dependent on VEGF-A but not VEGF-C/D, we sought to determine if macrophages expressed VEGF-A during infection. Transgenic mice expressing GFP under the proximal VEGF-A promoter (pVEGF-A–GFP) were infected with HSV-1, harvested at days 1, 3, and 5 PI, and examined for colocalization of GFP with the pan-leukocyte marker CD45. Although both CD45 and GFP+ cells were present, expression of GFP by CD45+ cells was not observed (Fig. 5 A), indicating that leukocytes were not an appreciable source of VEGF-A during HSV-1 infection.
Figure 5.
Macrophages are not involved in HSV-1–induced lymphangiogenesis. (A) Mice expressing GFP under the proximal human VEGF-A promoter were infected with HSV-1 and examined for expression of GFP and the pan-leukocyte marker CD45. No colocalization between GFP reporter and CD45 was observed, despite the observation of numerous CD45 cells as well as GFP+ cells, indicating that leukocytes, including macrophages, are not an appreciable source of VEGF-A during HSV-1 infection. Shown is an image of a cornea at day 5 PI representative of two experiments. n = 6. The images were acquired with a 400× objective. Bars, 50 µm. (B) Macrophages were depleted by subconjunctival injection with either clodronate or control PBS containing liposomes. At day 5 PI, corneal F4/80+ macrophages were quantitated by flow cytometry in control and clodronate-treated groups. Clodronate treatment significantly reduced the number of corneal F480+ cells (*, P < 0.05). (C) Corneas were examined for expression of the lymphatic vessel antigen LYVE-1 and quantified as a measure of lymphatic vessel area. No significant differences in lymphatic vessel area or structure (not depicted) were observed between control and clodronate-treated groups. B and C are summaries of two experiments. Error bars represent the SEM based on the results of each cornea sample summarized for both experiments. n = 6. (D) Chimeric mice expressing GFP in bone marrow–derived cells were infected with HSV-1. Corneas were examined for expression of GFP (green) and LYVE-1 (red) to detect possible structural contributions from bone marrow–derived cells. However, no appreciable structural contribution was observed. Data are representative of two experiments. n = 8 animals. Bars, 100 µm.
Furthermore, although macrophage reduction via administration of clodronate liposomes significantly reduced the absolute numbers of corneal F4/80+CD45HI cells detected via flow cytometry (79 ± 23 cells in control-treated versus 25 ± 8 cells in clodronate liposome-treated eyes; Fig. 5 B), HSV-1–induced lymphangiogenesis was not affected as determined by measurement of LYVE-1+ percentage area in random fields of view (Fig. 5 B, 5.5% ± 0.9% vs. 5.1% ± 0.9% in PBS-treated vs. clodronate liposome–treated groups, respectively). To determine if bone marrow–derived cells made a structural contribution to lymphatic vessels, chimeric mice with bone marrow cells expressing GFP under the chicken β-actin were infected with HSV-1. Despite extensive infiltration of GFP+ bone marrow–derived cells into the cornea, appreciable colocalization between GFP and LYVE-1 was not observed (Fig. 5 D). This indicates that bone marrow–derived cells do not transdifferentiate to lymphatic endothelial cells after corneal HSV-1 infection. Collectively, evidence of the lack of VEGF-A expression by leukocytes after HSV-1 infection, lack of impact through macrophage reduction via clodronate administration, or of a bone marrow–derived structural contribution to lymphatic vessels suggest that neither macrophages nor other leukocyte populations are likely to play an appreciable direct and prolymphangiogenic role during acute HSV-1–induced lymphangiogenesis.
The predominant source of VEGF-A during HSV-1 infection is HSV-1–infected corneal epithelial cells
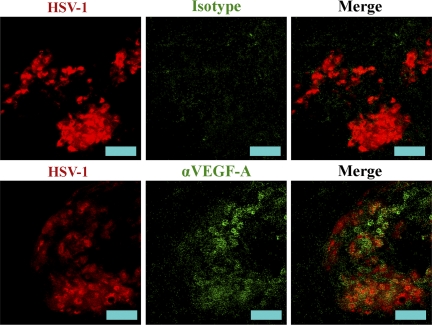
The lack of a role for macrophages, as well as the observation that VEGF-A expression is highest at day 1 PI (Fig. 4, A and B), which precedes leukocyte infiltration into the cornea after HSV-1 infection (Kim et al., 2004; Biswas et al., 2006), suggests that resident corneal cells may be a source of VEGF-A. Corneas were examined for expression of VEGF-A and HSV-1 antigen at days 1, 3, and 5 PI. Extensive colocalization of VEGF-A and HSV-1 antigen was observed. Furthermore, detectable VEGF-A was restricted to the vicinity of HSV-1 lesions (Fig. 6). Although colocalization was observed, there were areas which did not appear to overlap. However, most VEGF-A isoforms bind extracellular matrix, and VEGF-A would also be expected to be bound to VEGFR-positive cells. Therefore, localization of VEGF-A does not indicate the site of its source. However, the observation that VEGF-A concentrations were greatest at sites of HSV-1 lesions was consistent with the hypothesis that cell populations in the vicinity of these lesions were the dominant source of VEGF-A during acute infection.
Figure 6.
VEGF-A expression is localized to HSV-1 lesions. To gauge localization of the source of VEGF-A during HSV-1 infection, corneas were stained for HSV-1 antigen (red) and VEGF-A or isotypic control (green). VEGF-A staining was localized to sites of HSV-1 lesions. Representative images are shown of two experiments. n = 6 mice. Images were acquired with a 200× objective. Bars, 50 µm.
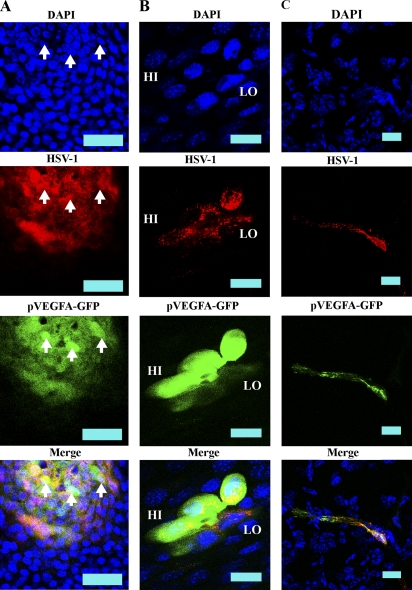
To clarify the cell source, pVEGF-A–GFP mice were infected with 105 HSV-1 and expression of GFP and HSV-1 antigen was examined. GFP reporter expression was localized exclusively to cells coexpressing HSV-1 antigen at day 1, 3, and 5 PI (Fig. 7 A, day 1 PI) and was primarily observed in corneal epithelial cells. However, an occasional HSV-1–infected stromal cell also expressed GFP VEGF-A reporter (Fig. 7 C). VEGF-A reporter expression could be categorized between either GFP HI or LO with differential HSV-1 antigen expression between the two groups (Fig. 7 B). In GFP HI cells, HSV-1 antigen expression was slightly lower than that observed in GFP LO cells, and HSV-1 antigen staining was primarily perinuclear in these cells. In contrast, GFP LO cells typically expressed high levels of HSV-1 antigen, which was diffuse throughout the cytoplasm. At this point, the delineation between high and low GFP-expressing cells is unclear and may be the result of differential stages within the cycle of viral replication. However, both GFP HI and LO cells coexpressed HSV-1 antigen and, thus, the sole source of observable reporter expression was HSV-1–infected cells. Therefore, the contribution from leukocytes or other uninfected cell populations to VEGF-A expression appear negligible.
Figure 7.
The dominant source of VEGF-A during HSV-1 infection is virus-infected cells. To determine the source of VEGF-A, pVEGF-GFPs were infected with HSV-1. For VEGF-A transcription, we used a reporter mouse line expressing GFP under the proximal VEGF-A promoter. Corneas were harvested at days 1, 3, and 5 PI with HSV-1. (A) GFP (green) was visualized using secondary detection along with HSV-1 antigen (red), shown here from a cornea at 24 h PI. Reporter expression was observed exclusively in HSV-1 antigen-positive cells. Although cells expressing GFP reporter were positive for HSV-1 antigen, in cells expressing the highest levels of GFP, HSV-1 antigen staining was predominantly perinuclear, possibly reflecting a particular stage in viral replication. GFP HI cells are highlighted by arrows. Images were acquired with a 400× objective. Data are representative of two experiments. n = 6. Bars, 50 µm. (B) High-objective (600×) image of GFP HI and LO expressing HSV-1 antigen-positive cells. Bars, 20 µm. (C) Although the vast majority of cells infected with HSV-1 through day 5 PI are corneal epithelial cells, stromal cells expressing HSV-1 antigen also expressed GFP reporter for VEGF-A. Bars, 20 µm.
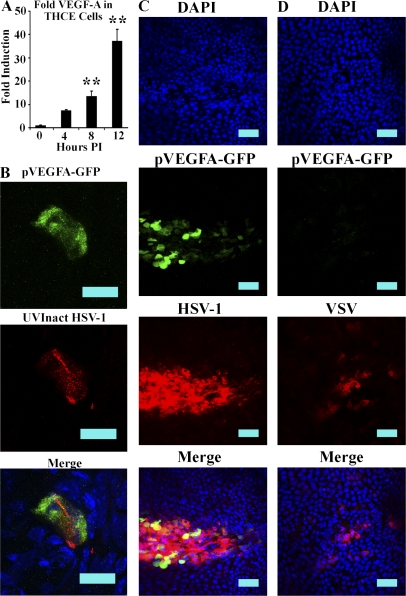
To determine if HSV-1 was also capable of driving VEGF-A expression in human corneal epithelial cells, tert-immortalized human corneal epithelial (THCE) cells were analyzed by real time RT-PCR after infection with 3 MOI HSV-1. To control for variable effects of HSV-1 infection on housekeeping gene expression, VEGF-A complementary DNA (cDNA) was measured relative to the three housekeeping genes: β-actin, tata binding protein, and GAPDH. The geometric mean of fold induction of VEGF-A relative to each housekeeping gene was then calculated and taken as the fold induction of the respective sample. Significant up-regulation of VEGF-A occurred as early as 8 h PI (Fig. 8 A, P < 0.01) and exhibited ∼37-fold induction relative to uninfected cell expression of VEGF-A by 12 h PI (Fig. 8 A, P < 0.01).
Figure 8.
HSV-1 induces VEGF-A up-regulation in THCEs and up-regulation of VEGF-A is HSV-1 specific. (A) THCE cells were infected with 3 MOI of HSV-1 and then analyzed by real-time RT-PCR for the expression of VEGF-A relative to the housekeeping genes β-actin, TBP, and PPIA. The fold induction of VEGF-A at each time point was calculated relative to each respective housekeeping gene and then the geometric mean was taken as the fold induction of VEGF-A per sample. Data are a summary of two experiments, with a total of n = 6 per time point. Data are expressed as mean ± SEM fold induction. **, P < 0.01, comparing infected to uninfected cells. (B) pVEGF-A–GFP transgenic mice were infected with a dose of replication incapable UV-inactivated HSV-1 corresponding to 105 PFU HSV-1 before inactivation. At 24 h PI, corneas were harvested and examined for localization of GFP relative to HSV-1 antigen. Cells expressing GFP costained for HSV-1 antigen expression, whereas neighboring bystander cells did not. Data are representative of two experiments with a total of n = 6. Bars, 20 µm. (C and D) Although HSV-1 induced up-regulation of GFP in pVEGF-A–GFP mice (C), infection with VSV did not (D), indicating that the up-regulation of VEGF-A after HSV-1 infection may be the result of an HSV-1–specific pathway. Bars, 50 µm. Data are representative of two experiments with a total of n = 6.
To control for the possibility that GFP VEGF-A reporter-expressing cells in pVEGF-A–GFP mice may express VEGF-A before infection as a result of other stimuli, pVEGF-A–GFP transgenic mice were treated with inocula of UV-inactivated nonreplicating HSV-1 (corresponding to 105 PFU HSV-1 before UV inactivation). After UV inactivation, plaque assays confirmed complete inactivation of virus (unpublished data). Infection with UV-inactivated virus still induced expression of GFP (Fig. 8 B). However, both GFP and HSV-1 antigen expression per cell was lower than that observed after infection with infectious virus. Importantly, bystander cells did not express GFP reporter.
Next, we sought to determine if HSV-1–induced VEGF-A expression was reflective of a general response to viral infection or HSV-1 selective. We compared the expression of GFP in pVEGF-A–GFP transgenic mice infected with 105 PFU of either HSV-1 or VSV. Although HSV-1 induced high level reporter expression (Fig. 8 C), we did not observe GFP expression in VSV antigen-positive cells (Fig. 8 D). Although this assessment is not comprehensive, it is intriguing to note that a DNA virus (HSV-1) elicits lymphangiogenesis whereas an RNA virus (VSV) does not. Whether this relationship holds for other DNA and RNA viruses is unknown at this time.
DISCUSSION
Several studies have clarified the regulation of inflammatory lymphangiogenesis during wound healing, chronic bacterial infection, and Toll-like receptor (TLR) ligand administration. To our knowledge, before this study no attempts had been made to clarify the existence or mechanism of inflammatory lymphangiogenesis during chronic inflammation as a result of viral infection. Inflammatory lymphangiogenesis during wound healing and bacterial infection depends on macrophage infiltration for subsequent production of the prolymphangiogenic cytokines VEGF-C and D (Cursiefen et al., 2003. 2004b; Watari et al., 2008; Kataru et al., 2009). However, HSV-1–induced lymphangiogenesis differed in both the absence of the requirement for macrophage recruitment and dependence on VEGF-C or D. Rather, HSV-1–elicited lymphangiogenesis was dependent on VEGF-A derived from HSV-1–infected cells.
The basis for a lack of a role for VEGFR-3 ligands is currently unclear. Regulation of VEGF-C and D expression is poorly studied but NF-κB activation is crucial to both VEGF-C and D expression and is up-regulated during HSV-1 infection (Chilov et al., 1997; Amici et al., 2001; Taddeo et al., 2003; Hsieh et al., 2004; Watari et al., 2008). Yet, we did not observe up-regulation of VEGF-C or D transcript or of any impact from competitive inhibition using VEGFR3-Fc despite abundant expression of VEGFR-3 on HSV-1–induced lymphatic vessels. The primary sources of VEGFR-3 ligands during inflammation are infiltrating macrophages (Cursiefen et al., 2003, 2004b; Kerjaschki, 2005; Cursiefen, 2007; Watari et al., 2008; Kataru et al., 2009). During HSV-1 infection, macrophage infiltration is extensive. However, these cells were irrelevant to HSV-1–induced lymphangiogenesis, suggesting that the function and activity of macrophages differed between macrophages infiltrating the cornea during HSV-1 infection as opposed to wound healing or other sources of inflammation (Kubota et al., 2009). Hypoxia functions to repress VEGF-C production while elevating VEGF-A expression (Gunningham et al., 2001). But the possibility of hypoxic corneal conditions alone do not provide a satisfactory explanation, as hypoxia induces macrophage production of VEGF-A (Ray et al., 2009), yet no VEGF-A reporter expression by CD45+ cells was observed during HSV-1 infection. We also did not observe a suppressive effect of HSV-1 infection on VEGF-C up-regulation after suture placement (Fig. 4 C). Thus, it appears that the lack of VEGF-C up-regulation by HSV-1 infection is a result of the absence of VEGF-C–inducing signaling pathways activated during other forms of inflammation rather than a VEGF-C–suppressive pathway activated by HSV-1 infection.
VEGF-A/VEGFR-2–induced lymphatic vessels differ in structure and function from those induced by VEGFR-3 ligands, exhibiting a relatively dilated, leaky, and poorly functional phenotype (Nagy et al., 2002; Hong et al., 2004b; Kajiya et al., 2006). HSV-1–elicited lymphatic vessels are likely to be poor transporters of antigen compared with vessels induced by VEGF-C or D. However, they are still capable of draining soluble antigen from the cornea to the lymph nodes. FITC-labeled high molecular mass dextran injected intrastromally into the cornea of HSV-1–infected mice is rapidly transported through lymphatic vessels to draining lymph nodes (Figs. S2). HSV-1–induced lymphatic vessels remained intact and functional well beyond resolution of infection. The normal human cornea is immunologically privileged, but during HSK, chronic inflammation mediated by the host immune response results in blinding corneal opacity (Carr et al., 2001; Biswas et al., 2006). Part of the basis for corneal immunological privilege is the absence of lymphatic vessels (Niederkorn, 2003; Streilein, 2003; Cursiefen, 2007). The lack of presentation of eye antigens during normal development prevents the induction of T cell anergy (Lambe et al., 2007). Lymphangiogenesis during ocular HSV-1 infection may be a driving force behind the loss of immunological privilege by allowing the transport of corneal antigens. Studies assessing the impact of corneal lymphatics on antigen presentation and immunological privilege are ongoing. Lymphangiogenesis may also negatively affect standard treatment for HSK. In severe cases, patients may require corneal transplant to restore vision. However, corneal transplant rejection rates in patients with HSK are unusually high (Larkin, 1998). The presence of corneal lymphatic vessels is associated with an elevated risk of graft rejection (Larkin, 1998; Cursiefen et al., 2003, 2004a; Regenfuss et al., 2008) that is likely the result of enhanced antigen transport to draining lymph nodes, which are required for the induction of graft rejection (Yamagami and Dana, 2001).
An earlier study of HSK-associated hemangiogenesis demonstrated that during corneal HSV-1 infection, VEGF-A was predominantly localized to the corneal epithelium at sites of HSV-1 lesions (Biswas et al., 2006), which is consistent with our observations. However, this study asserted that uninfected corneal epithelial cells in close proximity to HSV-1–infected cells were the predominant source of VEGF-A. This conclusion was based on the observation that VEGF-A+ cells were localized immediately neighboring on cells expressing a GFP reporter under the promoter for HSV-1 glycoprotein C (gC). However, gC is a true late gene and gC transcription does not commence until the completion of viral DNA synthesis (Yamamoto et al., 2006). Thus, a lag time exists after viral infection before detection of reporter expression (Zabierowski and DeLuca, 2004). Should VEGF-A expression occur in HSV-1–infected cells before gC expression, the use of this reporter to detect infected cells would lead to the erroneous conclusion that uninfected cells were the primary source of VEGF-A. In addition, most VEGF-A isoforms contain extracellular matrix binding domains. Localization of VEGF-A is not restricted to sites of VEGF-A expression (Yamazaki and Morita, 2006), and a concentration gradient from sources to VEGFR is required for exertion of proangiogenic or prolymphangiogenic effects (Yamazaki and Morita, 2006). To avoid these issues we used reporter mice expressing GFP under the proximal VEGF-A promoter to identify VEGF-A–expressing cells as well as polyclonal antibody against whole HSV-1 to identify HSV-1–infected cells. Infected primarily corneal epithelial cells at time points assayed were the sole detectable source of GFP reporter.
Studies to address the mechanism of VEGF-A up-regulation are ongoing. Theoretically, HSV-1–associated TLR ligands acting through either MyD88 or TRIFF adaptor proteins may participate, as activation of MyD88 by several different TLR ligands up-regulates VEGF-A expression (Pinhal-Enfield et al., 2003; Varoga et al., 2006; Macedo et al., 2007; Koff et al., 2008). MyD88 deficiency also reduces VEGF-A levels during the early course (day 2 PI) of corneal HSV-1 infection (Sarangi et al., 2007). Although no significant difference in VEGF-A levels between WT and MyD88-deficient animals is found by day 4 PI (Sarangi et al., 2007), a time point at which VEGF-A production is still dominated by HSV-1–infected cells, interpretation may be complicated by elevated viral replication in MyD88-deficient animals that may up-regulate VEGF-A through otherwise less dominant compensatory pathways. A role for TRIFF-dependent TLR signaling would appear less likely. Even though the TRIFF-dependent TLR, TLR3, is believed to be the primary TLR involved in recognition of HSV-1 infection (Zhang et al., 2007), activation of TRIFF via TLR3 ligands inhibits VEGF-A expression (Kleinman et al., 2008). However, inhibition of VEGF-A expression by TRIFF activation requires downstream expression of IFN-γ (Kleinman et al., 2008). HSV-1 ICP0 inhibits signaling downstream of IFN-γ (Härle et al., 2002b), possibly alleviating VEGF-A repression.
Alternatively, VEGF-A up-regulation may be TLR independent. The VEGF-A promoter is well characterized, and HSV-1 infection modulates the activity/abundance of three transcription factors involved in transcriptional regulation of VEGF-A: STAT3, Sp1, and EGR-1 (Imbalzano et al., 1991; Kim and DeLuca, 2002, Rajcáni et al., 2004; Pagès and Pouysségur, 2005; Biswas et al., 2006; Iwahori et al., 2007; Chen et al., 2008). The impact of STAT3 may be indirect through paracrine activation by IL-6 produced in HSV-1–infected cells (Biswas et al., 2006). However, Sp1 and EGR-1 both exhibit early changes in abundance and activity after infection (Imbalzano et al., 1991; Kim and DeLuca, 2002, Rajcáni et al., 2004; Pagès and Pouysségur, 2005; Iwahori et al., 2007; Chen et al., 2008).
VEGF-A antagonists are already in use for the treatment of various malignancies and other disease processes. Our results suggest the possibility for the extension of their utility to the management of HSK. We have observed up-regulated expression of VEGF-A by HSV-1–infected skin epithelial cells (unpublished data). Identification of HSV-1–infected cells as a source of VEGF-A leads to more questions regarding the impact of this cytokine on other facets of HSV-1 disease. DC maturation is inhibited during HSV-1 infection (Salio et al., 1999) and VEGF-A inhibits DC maturation and function via VEGFR1 signaling (Soker et al., 1998; Dikov et al., 2005; Fricke et al., 2007). VEGF-A also has profound affects on neuronal and blood vascular systems that may be clinically relevant. Neuronal axons are guided to VEGF-A sources through VEGF-A interaction with neuropilins and VEGFRs (Neufeld et al., 2002; Schwarz et al., 2004; Yu et al., 2008). Sensory neurons feeding the site of primary HSV-1 infection are the ultimate target of the virus, as sensory neuron infection is required for the virus to establish latency within the trigeminal ganglia (Taylor et al., 2002). Thus, VEGF-A blockade may have profound effects on the course of HSV-1 disease, impacting both the initial course of disease and immunological responses to it. It remains to be seen whether HSV-1 infection up-regulates the expression of the VEGFR-1 binding VEGF family members VEGF-B and PlGF. Delayed up-regulation of expression of either of these cytokines could provide an explanation for the disparate time course between lymph and hemangiogenesis during HSV-1. Although blood vessels express VEGFR-1, which signals synergistically with VEGFR-2 (Fischer et al., 2007), lymphatic vessels do not express VEGFR-1 (Hong et al., 2004a).
In summary, this paper is the first in vivo study that addresses inflammatory lymphangiogenesis during viral infection. In contrast to established models of inflammatory lymphangiogenesis, HSV-1–induced lymphangiogenesis was not dependent on the VEGFR-3 ligands VEGF-C and D or on macrophage infiltration. Instead, lymphangiogenesis was dependent on VEGF-A, and VEGF-A production by HSV-1–infected cells was not a general response to viral infection, as VSV–infected cells did not cause VEGF-A expression. Lymphatic vessels persisted well past resolution of infection and may contribute to chronic inflammation and blindness in susceptible patients.
MATERIALS AND METHODS
Immunofluorescence microscopy and quantitative analysis of lymphangiogenesis.
Corneas were prepared and stained for specific antigens as described previously (Wuest et al., 2006a). LYVE-1 expression was quantitated by taking 100× objective images of corneas with the top of the field localized to include lymphatic vessels in the limbus of the eye as well as any lymphatic vessels extending into the cornea. The cornea was oriented such that the corneal area included was equivalent between individual corneas by using limbal lymphatics and the delineation between the cornea and limbus as reference points. Images were analyzed with MetaMorph Imaging Suite v7.0 (MDS Analytical Technologies), with the lymphatic vessel area defined as the percentage of LYVE-1+ pixels divided by the total number of pixels per field of view. The sources of antibodies used are the following: rabbit anti–HSV-1, rat anti–mouse CD45, goat anti–mouse VEGFR-2, rabbit anti–mouse LYVE-1 (Abcam), goat anti-GFP, FITC rat anti–mouse CD31 (AbD Serotec), Alexa Fluor 546 goat anti–rabbit, Alexa Fluor 568 rabbit anti–goat, Alexa Fluor 647 chicken anti–rabbit, (Invitrogen), goat anti–mouse VEGF-A (R&D Systems), FITC rat anti–mouse F4/80 (BD), goat anti–mouse VEGFR-3 (Thermo FIsher Scientific), DyLight 549 donkey anti–rabbit, DyLight 549 anti–rat, and FITC bovine anti–goat (Jackson ImmunoResearch Laboratories). Images were taken using an epifluorescence/confocal laser-scanning microscope (IX81-FV500; Olympus).
Flow cytometry and cytokine measurement.
Corneas and lymph nodes were processed for flow cytometry and quantitation of the absolute number of macrophages or FITC-Dextran–labeled cells was performed as previously described (Wuest et al., 2006b; Wuest and Carr, 2008). FITC-Dextran levels in the draining lymph nodes was assessed in terms of arbitrary units using the multiple of total FITC-Dextran+ events times their mean fluorescence intensity. VEGF-A levels were measured by cytokine bead array using a Bio-plex suspension array system (Bio-Rad Laboratories) as previously described (Wuest and Carr, 2008).
Macrophage depletion and inhibition of lymphangiogenesis.
PBS- and clodronate-containing liposomes were the gift of N. van Rooijen (Vrije Universiteit Medical Center, Amsterdam, Netherlands). Corneal macrophages were depleted using clodronate liposomes. Mice were given subconjunctival injections of 10 µL of either clodronate- or PBS-containing liposomes at days −3, 0, 2, or 4 relative to the time of infection. Corneas were harvested at day 5 PI and the number of F4/80+ cells was determined as well as lymphatic vessel area. To competitively inhibit the prolymphangiogenic VEGF family members VEGF-A, C, or D, mice were injected subconjunctivally with 10 µl of recombinant human IgG1, VEGFR2-Fc, or VEGFR3-Fc (R&D Systems), all at a concentration of 100 µg/ml in sterile 0.1% BSA in PBS. The injections were performed at time points of days 0, 2, and 4 relative to the time of infection with HSV-1. For experiments using antibody neutralization of VEGF-A and VEGFR-2, monoclonal rat anti–mouse VEGFR-2 (clone AP-MAB-0701; Angio-Proteomie), monoclonal rat anti–mouse VEGF-A (clone 2G11-2A05; BioLegend), or purified rat IgG (Angio-Proteomie) were administered by 10-µl subconjunctival injections at days 0, 2, and 4, all at a concentration of 100 µg/ml in sterile 0.1% BSA in PBS. Corneas were harvested at day 5 PI and lymphatic vessel area was determined. For study of VEGFR-3–dependent lymphangiogenesis in response to corneal suture placement, a single number 7 braided silk suture was placed in the center of the cornea of C57BL/6 mice. Suture versus nonsutured animals received either control IgG or VEGFR3-Fc using the same doses and dosing schedule as with experiments using HSV-1.
Dye transport assays.
FITC-labeled dextran (2,000 kD lysine fixable FITC-Dextran) was injected into the cornea intrastromally via glass needle in a volume of 1 µl at a concentration of 10 mg/ml in PBS, using a gas-powered microinjection system (MDI) under an ophthalmic surgical microscope (Carl Zeiss , Inc.) at days 0 or 5 PI with HSV-1. Whole eye was harvested at 1 h after injection and fixed in 4% paraformaldehyde in PBS for 1 h. Lymph nodes were fixed for 30 min in 4% paraformaldehyde, soaked in 30% sucrose overnight, and then snap frozen before sectioning. Nuclei were stained using Vectashield DAPI containing mounting media (Vector Laboratories) and images taken via confocal microscopy.
HSV-1 infection and mice.
Male C57BL/6 and C57BL/6 mice expressing GFP under the chicken β-actin promoter were purchased from The Jackson Laboratory. Anesthetized mice were infected with HSV-1 by scarifying the cornea with a 25-gauge needle and applying 105 PFU HSV-1 per cornea as indicated. Transgenic reporter mice expressing GFP under the proximal VEGF-A promoter were the gift of B. Seed (Harvard University, Boston, MA) and were constructed on an FVB background as previously described (Fukumura et al., 1998). Reporter expression was visualized by secondary detection of GFP. Mice used were between 6 wk and 6 mo old and age-matched controls were used in each experiment. HSV-1 virus stocks were propagated using vero cells and maintained at a concentration of 1 × 109 PFU/ml at −80°C. To generate UV-inactivated virus, HSV-1 was placed in open plastic Petri dishes on ice and exposed to 120,000 µm/cm2 for 1 min using a Spectrolinker XL-1000 (Spectronics). Inactivation was verified by plaque assay. Animal protocols were approved by the University of Oklahoma Health Sciences Center and Dean McGee Eye Institute institutional animal care and use committees.
Bone marrow chimeras.
Bone marrow chimeras were created by irradiating WT mice with two 600-rad doses of γ irradiation spaced 4 h apart. Irradiated mice were then IV-injected with 3 × 106 bone marrow cells from mice expressing GFP under the β-actin promoter. The injected bone marrow cells were allowed 10 wk to reconstitute the hematopoietic compartment and chimerism was verified by flow cytometry.
Real-time RT-PCR.
Corneas were harvested and RNA was isolated at indicated time points PI using Trizol as per the manufacturer's instructions (Invitrogen). After RNA isolation, 2 µg RNA per sample was converted to cDNA using an RT system (Promega) using random primers according to the manufacturer's instruction. Samples were then analyzed via real-time PCR using Sybr Green supermix (Bio-Rad Laboratories) via an iCycler (Bio-Rad Laboratories) as previously described (Härle et al., 2002a). The abundance of VEGF-A/C/D cDNA relative to the housekeeping gene β-actin was calculated as 2−ΔΔCt. For analysis of VEGF-A up-regulation using THCE cells, VEGF-A fold induction was determined using a panel of three housekeeping to control for effects of HSV-1 on housekeeping gene expression. VEGF-A cDNA relative to β-actin, TBP, or GAPDH was calculated as described for β-actin, and the geometric mean of the threefold induction values was taken as being the individual fold induction for the respective sample. THCE cells were maintained in keratinocyte SFM (Invitrogen) supplemented with 0.15 ng/ml EGF and 0.25 µg/ml bovine pituitary extract. THCE cells were plated at densities of 3 × 105 cells per well in 12-well plates and then infected with 3 MOI HSV-1 the next day before harvesting RNA at the indicated time point using Trizol. Primers were purchased from Sigma-Aldrich and sequences used were as follows: Mu VEGF-A forward, 5′-CTGCTGTACCTCCACCATGC-3′; Mu VEGF-A reverse, 5′-TCACTTCATGGGACTTCTGCTCT-3′; Mu VEGF-C forward, 5′-CTGGGAAATGTGCCTGTGAATG-3′; Mu VEGF-C reverse, 5′-ATTCGCACACGGTCTTCTGTAAC-3′; Mu VEGF-D forward, 5′-CAAGACGAGACTCCACTGCC-3′; Mu VEGF-D reverse, 5′-GCACTCACAGCGATCTTCATC-3′; Mu β-actin forward, 5′-CTTCTACAATGAGCTGCGTGTG-3′; Mu β-actin reverse, 5′-TTGAAGGTCTCAAACATGATCTGG-3′; Hu VEGF-A forward, 5′-AGGAGGAGGGCAGAATCATCA-3′; Hu VEGF-A reverse, 5′-CTCATTGGATGGCAGTAGCT-3′; Hu β-actin forward, 5′-AGCCTCGCCTTTGCCGA-3′; Hu β-actin reverse, 5′-CATGTCGTCCCAGTTGGTGAC-3′; Hu TBP forward, 5′-TGCACAGGAGCCAAGAGTGAA-3′; Hu TBP reverse, 5′-CACATCACAGCTCCCCACCA-3′; Hu PPIA forward, 5′-GTCAACCCCACCGTGTTCTT-3′; and Hu PPIA reverse, 5′-CTGCTGTCTTTGGGACCTTGT-3′.
Statistics.
Comparisons between multiple treatment groups were performed using one-way analysis of variance and Tukey's multiple comparison tests. All statistical analysis was performed with GBSTAT (Dynamic Micro Systems).
Online supplemental material.
Fig. S1 shows isotypic control antibody for anti-VEGFR2 and anti-VEGFR3 staining shown in Fig. 3. Fig. S2 shows corneal lymphatic vessel transport of soluble antigen in infected and uninfected mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091385/DC1.
Acknowledgments
The authors would like to thank Gabrielle Nguyen, Linh Sramek, and Min Zheng for their technical assistance. We would also like to thank Dr. Ashish Chintakuntlawar for initially performing corneal intrastromal injections.
This work was supported in part by National Institutes of Health grants EY018834 to D.J.J. Carr. T.R. Wuest was supported by the National Institute of Allergy and Infectious Disease training grant AI007633. Additional support includes P20 RR017703 and core grant EY12190.
The authors have no financial conflict of interest.
Footnotes
Abbreviations used:
- cDNA
- complementary DNA
- gC
- glycoprotein C
- HSK
- herpes simplex keratitis
- HSV-1
- herpes simplex virus 1
- PI
- post infection
- THCE
- tert-immortalized human corneal epithelial cell
- TLR
- Toll-like receptor
- VEGF
- vascular endothelial growth factor
- VEGFR
- VEGF receptor
- VSV
- vesicular stomatitis virus
References
- Amici C., Belardo G., Rossi A., Santoro M.G. 2001. Activation of IκB kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759–28766 10.1074/jbc.M103408200 [DOI] [PubMed] [Google Scholar]
- Baluk P., Tammela T., Ator E., Lyubynska N., Achen M.G., Hicklin D.J., Jeltsch M., Petrova T.V., Pytowski B., Stacker S.A., et al. 2005. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 115:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P.S., Banerjee K., Kinchington P.R., Rouse B.T. 2006. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp. Eye Res. 82:46–54 10.1016/j.exer.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Carr D.J., Härle P., Gebhardt B.M. 2001. The immune response to ocular herpes simplex virus type 1 infection. Exp. Biol. Med. (Maywood). 226:353–366 [DOI] [PubMed] [Google Scholar]
- Carr D.J.J., Austin B.A., Halford W.P., Stuart P.M. 2009. Delivery of Interferon-γ by an adenovirus vector blocks herpes simplex virus Type 1 reactivation in vitro and in vivo independent of RNase L and double-stranded RNA-dependent protein kinase pathways. J. Neuroimmunol. 206:39–43 10.1016/j.jneuroim.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., Yao H.W., Chen I.T., Shieh B., Li C., Chen S.H. 2008. Suppression of transcription factor early growth response 1 reduces herpes simplex virus lethality in mice. J. Clin. Invest. 118:3470–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilov D., Kukk E., Taira S., Jeltsch M., Kaukonen J., Palotie A., Joukov V., Alitalo K. 1997. Genomic organization of human and mouse genes for vascular endothelial growth factor C. J. Biol. Chem. 272:25176–25183 10.1074/jbc.272.40.25176 [DOI] [PubMed] [Google Scholar]
- Cueni L.N., Detmar M. 2008. The lymphatic system in health and disease. Lymphat. Res. Biol. 6:109–122 10.1089/lrb.2008.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C. 2007. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy. 92:50–57 10.1159/000099253 [DOI] [PubMed] [Google Scholar]
- Cursiefen C., Chen L., Dana M.R., Streilein J.W. 2003. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 22:273–281 10.1097/00003226-200304000-00021 [DOI] [PubMed] [Google Scholar]
- Cursiefen C., Cao J., Chen L., Liu Y., Maruyama K., Jackson D., Kruse F.E., Wiegand S.J., Dana M.R., Streilein J.W. 2004a. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest. Ophthalmol. Vis. Sci. 45:2666–2673 10.1167/iovs.03-1380 [DOI] [PubMed] [Google Scholar]
- Cursiefen C., Chen L., Borges L.P., Jackson D., Cao J., Radziejewski C., D'Amore P.A., Dana M.R., Wiegand S.J., Streilein J.W. 2004b. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113:1040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikov M.M., Ohm J.E., Ray N., Tchekneva E.E., Burlison J., Moghanaki D., Nadaf S., Carbone D.P. 2005. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J. Immunol. 174:215–222 [DOI] [PubMed] [Google Scholar]
- Fischer C., Jonckx B., Mazzone M., Zacchigna S., Loges S., Pattarini L., Chorianopoulos E., Liesenborghs L., Koch M., De Mol M., et al. 2007. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 131:463–475 10.1016/j.cell.2007.08.038 [DOI] [PubMed] [Google Scholar]
- Fricke I., Mirza N., Dupont J., Lockhart C., Jackson A., Lee J.H., Sosman J.A., Gabrilovich D.I. 2007. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 13:4840–4848 10.1158/1078-0432.CCR-07-0409 [DOI] [PubMed] [Google Scholar]
- Fukumura D., Xavier R., Sugiura T., Chen Y., Park E.C., Lu N., Selig M., Nielsen G., Taksir T., Jain R.K., Seed B. 1998. Tumor induction of VEGF promoter activity in stromal cells. Cell. 94:715–725 10.1016/S0092-8674(00)81731-6 [DOI] [PubMed] [Google Scholar]
- Galea I., Bechmann I., Perry V.H. 2007. What is immune privilege (not)? Trends Immunol. 28:12–18 10.1016/j.it.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Gunningham S.P., Currie M.J., Han C., Turner K., Scott P.A., Robinson B.A., Harris A.L., Fox S.B. 2001. Vascular endothelial growth factor-B and vascular endothelial growth factor-C expression in renal cell carcinomas: regulation by the von Hippel-Lindau gene and hypoxia. Cancer Res. 61:3206–3211 [PubMed] [Google Scholar]
- Härle P., Cull V., Agbaga M.P., Silverman R., Williams B.R., James C., Carr D.J.J. 2002a. Differential effect of murine alpha/beta interferon transgenes on antagonization of herpes simplex virus type 1 replication. J. Virol. 76:6558–6567 10.1128/JVI.76.13.6558-6567.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härle P., Sainz B., Jr., Carr D.J., Halford W.P. 2002b. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology. 293:295–304 10.1006/viro.2001.1280 [DOI] [PubMed] [Google Scholar]
- He J., Ichimura H., Iida T., Minami M., Kobayashi K., Kita M., Sotozono C., Tagawa Y.I., Iwakura Y., Imanishi J. 1999. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J. Interferon Cytokine Res. 19:609–615 10.1089/107999099313749 [DOI] [PubMed] [Google Scholar]
- Hong Y.K., Lange-Asschenfeldt B., Velasco P., Hirakawa S., Kunstfeld R., Brown L.F., Bohlen P., Senger D.R., Detmar M. 2004a. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 18:1111–1113 [DOI] [PubMed] [Google Scholar]
- Hong Y.K., Shin J.W., Detmar M. 2004b. Development of the lymphatic vascular system: a mystery unravels. Dev. Dyn. 231:462–473 10.1002/dvdy.20179 [DOI] [PubMed] [Google Scholar]
- Hsieh C.Y., Chen C.A., Chou C.H., Lai K.P., Jeng Y.M., Kuo M.L., Wei L.H. 2004. Overexpression of Her-2/NEU in epithelial ovarian carcinoma induces vascular endothelial growth factor C by activating NF-κB: implications for malignant ascites formation and tumor lymphangiogenesis. J. Biomed. Sci. 11:249–259 [DOI] [PubMed] [Google Scholar]
- Imbalzano A.N., Coen D.M., DeLuca N.A. 1991. Herpes simplex virus transactivator ICP4 operationally substitutes for the cellular transcription factor Sp1 for efficient expression of the viral thymidine kinase gene. J. Virol. 65:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahori S., Shirata N., Kawaguchi Y., Weller S.K., Sato Y., Kudoh A., Nakayama S., Isomura H., Tsurumi T. 2007. Enhanced phosphorylation of transcription factor sp1 in response to herpes simplex virus type 1 infection is dependent on the ataxia telangiectasia-mutated protein. J. Virol. 81:9653–9664 10.1128/JVI.00568-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R.K. 2003. Molecular regulation of vessel maturation. Nat. Med. 9:685–693 10.1038/nm0603-685 [DOI] [PubMed] [Google Scholar]
- Kajiya K., Hirakawa S., Detmar M. 2006. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am. J. Pathol. 169:1496–1503 10.2353/ajpath.2006.060197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpanen T., Alitalo K. 2008. Molecular biology and pathology of lymphangiogenesis. Annu. Rev. Pathol. 3:367–397 10.1146/annurev.pathmechdis.3.121806.151515 [DOI] [PubMed] [Google Scholar]
- Kataru R.P., Jung K., Jang C., Yang H., Schwendener R.A., Baik J.E., Han S.H., Alitalo K., Koh G.Y. 2009. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 113:5650–5659 10.1182/blood-2008-09-176776 [DOI] [PubMed] [Google Scholar]
- Kaye S., Choudhary A. 2006. Herpes simplex keratitis. Prog. Retin. Eye Res. 25:355–380 10.1016/j.preteyeres.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Kerjaschki D. 2005. The crucial role of macrophages in lymphangiogenesis. J. Clin. Invest. 115:2316–2319 10.1172/JCI26354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Regele H.M., Moosberger I., Nagy-Bojarski K., Watschinger B., Soleiman A., Birner P., Krieger S., Hovorka A., Silberhumer G., et al. 2004. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J. Am. Soc. Nephrol. 15:603–612 10.1097/01.ASN.0000113316.52371.2E [DOI] [PubMed] [Google Scholar]
- Kim D.B., DeLuca N.A. 2002. Phosphorylation of transcription factor Sp1 during herpes simplex virus type 1 infection. J. Virol. 76:6473–6479 10.1128/JVI.76.13.6473-6479.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Tang Q., Biswas P.S., Xu J., Schiffelers R.M., Xie F.Y., Ansari A.M., Scaria P.V., Woodle M.C., Lu P., Rouse B.T. 2004. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am. J. Pathol. 165:2177–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman M.E., Yamada K., Takeda A., Chandrasekaran V., Nozaki M., Baffi J.Z., Albuquerque R.J.C., Yamasaki S., Itaya M., Pan Y., et al. 2008. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 452:591–597 10.1038/nature06765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff J.L., Shao M.X., Ueki I.F., Nadel J.A. 2008. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 294:L1068–L1075 10.1152/ajplung.00025.2008 [DOI] [PubMed] [Google Scholar]
- Kubota Y., Takubo K., Shimizu T., Ohno H., Kishi K., Shibuya M., Saya H., Suda T. 2009. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med. 206:1089–1102 10.1084/jem.20081605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe T., Leung J.C.H., Ferry H., Bouriez-Jones T., Makinen K., Crockford T.L., Jiang H.R., Nickerson J.M., Peltonen L., Forrester J.V., Cornall R.J. 2007. Limited peripheral T cell anergy predisposes to retinal autoimmunity. J. Immunol. 178:4276–4283 [DOI] [PubMed] [Google Scholar]
- Larkin D.F.P. 1998. Corneal transplantation for herpes simplex keratitis. Br. J. Ophthalmol. 82:107–108 10.1136/bjo.82.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang T.J., Melton L.J., III, Daly P.J., Ilstrup D.M. 1989. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch. Ophthalmol. 107:1155–1159 [DOI] [PubMed] [Google Scholar]
- Macedo L., Pinhal-Enfield G., Alshits V., Elson G., Cronstein B.N., Leibovich S.J. 2007. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am. J. Pathol. 171:1774–1788 10.2353/ajpath.2007.061048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Ii M., Cursiefen C., Jackson D.G., Keino H., Tomita M., Van Rooijen N., Takenaka H., D'Amore P.A., Stein-Streilein J., et al. 2005. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115:2363–2372 10.1172/JCI23874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J.A., Vasile E., Feng D., Sundberg C., Brown L.F., Detmar M.J., Lawitts J.A., Benjamin L., Tan X., Manseau E.J., et al. 2002. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J. Exp. Med. 196:1497–1506 10.1084/jem.20021244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Cohen T., Shraga N., Lange T., Kessler O., Herzog Y. 2002. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc. Med. 12:13–19 10.1016/S1050-1738(01)00140-2 [DOI] [PubMed] [Google Scholar]
- Niederkorn J.Y. 2003. The immune privilege of corneal grafts. J. Leukoc. Biol. 74:167–171 10.1189/jlb.1102543 [DOI] [PubMed] [Google Scholar]
- Oliver G. 2004. Lymphatic vasculature development. Nat. Rev. Immunol. 4:35–45 10.1038/nri1258 [DOI] [PubMed] [Google Scholar]
- Paavonen K., Mandelin J., Partanen T., Jussila L., Li T.F., Ristimaki A., Alitalo K., Konttinen Y.T. 2002. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J. Rheumatol. 29:39–45 [PubMed] [Google Scholar]
- Pagès G., Pouysségur J. 2005. Transcriptional regulation of the vascular endothelial growth factor gene—a concert of activating factors. Cardiovasc. Res. 65:564–573 10.1016/j.cardiores.2004.09.032 [DOI] [PubMed] [Google Scholar]
- Pinhal-Enfield G., Ramanathan M., Hasko G., Vogel S.N., Salzman A.L., Boons G.J., Leibovich S.J. 2003. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am. J. Pathol. 163:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgrabinska S., Braun P., Velasco P., Kloos B., Pepper M.S., Skobe M. 2002. Molecular characterization of lymphatic endothelial cells. Proc. Natl. Acad. Sci. USA. 99:16069–16074 10.1073/pnas.242401399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajcáni J., Andrea V., Ingeborg R. 2004. Peculiarities of herpes simplex virus (HSV) transcription: an overview. Virus Genes. 28:293–310 10.1023/B:VIRU.0000025777.62826.92 [DOI] [PubMed] [Google Scholar]
- Ray P.S., Jia J., Yao P., Majumder M., Hatzoglou M., Fox P.L. 2009. A stress-responsive RNA switch regulates VEGFA expression. Nature. 457:915–919 10.1038/nature07598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenfuss B., Bock F., Parthasarathy A., Cursiefen C. 2008. Corneal (lymph)angiogenesis—from bedside to bench and back: a tribute to Judah Folkman. Lymphat. Res. Biol. 6:191–201 10.1089/lrb.2008.6348 [DOI] [PubMed] [Google Scholar]
- Salio M., Cella M., Suter M., Lanzavecchia A. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245–3253 [DOI] [PubMed] [Google Scholar]
- Sarangi P.P., Kim B., Kurt-Jones E., Rouse B.T. 2007. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J. Virol. 81:11128–11138 10.1128/JVI.01008-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q., Gu C., Fujisawa H., Sabelko K., Gertsenstein M., Nagy A., Taniguchi M., Kolodkin A.L., Ginty D.D., Shima D.T., Ruhrberg C. 2004. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 18:2822–2834 10.1101/gad.322904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.S., Robinson N.J. 2002. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 186:S3–S28 10.1086/343739 [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. 1998. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 92:735–745 10.1016/S0092-8674(00)81402-6 [DOI] [PubMed] [Google Scholar]
- Streilein J.W. 2003. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 74:179–185 10.1189/jlb.1102574 [DOI] [PubMed] [Google Scholar]
- Taddeo B., Luo T.R., Zhang W., Roizman B. 2003. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc. Natl. Acad. Sci. USA. 100:12408–12413 10.1073/pnas.2034952100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T.J., Brockman M.A., McNamee E.E., Knipe D.M. 2002. Herpes simplex virus. Front. Biosci. 7:d752–d764 10.2741/taylor [DOI] [PubMed] [Google Scholar]
- Varoga D., Paulsen F., Mentlein R., Fay J., Kurz B., Schütz R., Wruck C., Goldring M.B., Pufe T. 2006. TLR-2-mediated induction of vascular endothelial growth factor (VEGF) in cartilage in septic joint disease. J. Pathol. 210:315–324 10.1002/path.2059 [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Mempel T.R. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878 10.1038/nri1222 [DOI] [PubMed] [Google Scholar]
- Watari K., Nakao S., Fotovati A., Basaki Y., Hosoi F., Bereczky B., Higuchi R., Miyamoto T., Kuwano M., Ono M. 2008. Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem. Biophys. Res. Commun. 377:826–831 10.1016/j.bbrc.2008.10.077 [DOI] [PubMed] [Google Scholar]
- Wickham S., Carr D.J.J. 2004. Molecular mimicry versus bystander activation: herpetic stromal keratitis. Autoimmunity. 37:393–397 10.1080/08916930410001713106 [DOI] [PubMed] [Google Scholar]
- Wuest T.R., Carr D.J. 2008. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J. Immunol. 181:7985–7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest T., Austin B.A., Uematsu S., Thapa M., Akira S., Carr D.J.J. 2006a. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J. Neuroimmunol. 179:46–52 10.1016/j.jneuroim.2006.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest T., Farber J., Luster A., Carr D.J. 2006b. CD4+ T cell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection. Cell. Immunol. 243:83–89 10.1016/j.cellimm.2007.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami S., Dana M.R. 2001. The critical role of lymph nodes in corneal alloimmunization and graft rejection. Invest. Ophthalmol. Vis. Sci. 42:1293–1298 [PubMed] [Google Scholar]
- Yamamoto S., Deckter L.A., Kasai K., Chiocca E.A., Saeki Y. 2006. Imaging immediate-early and strict-late promoter activity during oncolytic herpes simplex virus type 1 infection and replication in tumors. Gene Ther. 13:1731–1736 10.1038/sj.gt.3302831 [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Morita T. 2006. Molecular and functional diversity of vascular endothelial growth factors. Mol. Divers. 10:515–527 10.1007/s11030-006-9027-3 [DOI] [PubMed] [Google Scholar]
- Yu C.Q., Zhang M., Matis K.I., Kim C., Rosenblatt M.I. 2008. Vascular endothelial growth factor mediates corneal nerve repair. Invest. Ophthalmol. Vis. Sci. 49:3870–3878 10.1167/iovs.07-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabierowski S., DeLuca N.A. 2004. Differential cellular requirements for activation of herpes simplex virus type 1 early (tk) and late (gC) promoters by ICP4. J. Virol. 78:6162–6170 10.1128/JVI.78.12.6162-6170.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P., Segal D., Sancho-Shimizu V., Lorenzo L., Puel A., et al. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 317:1522–1527 10.1126/science.1139522 [DOI] [PubMed] [Google Scholar]