Abstract
Background
The objective of this study was to ascertain patient preferences for treatment of HCV.
Methods
We recruited consecutive patients eligible for treatment of HCV and used Adaptive Conjoint Analysis (ACA), a hybrid approach of conjoint analysis that uses both self-explicated ratings and pairwise comparisons, to elicit preferences for pegylated-interferon and ribavirin. We examined the association between patient characteristics and treatment preferences using the Mann-Whitney U test and chi-square statistic for continuous and categorical variables respectively and subsequently calculated adjusted odds ratios and 95% confidence intervals using logistic regression.
Results
140 subjects completed the ACA task. The mean (±SD) age of the sample was 51 ± 8, 85% were male, and 59% White. When described as being associated with mild side effects, 67% (N=94) of subjects’ preferred treatment for HCV. The percentage of subjects preferring therapy decreased to 51% (N=72) when it was described as being associated with severe side effects. Preferences for treatment of HCV were stronger among subjects with a higher perceived risk of developing cirrhosis, more severe underlying liver disease, and worse HCV-related quality of life. Subjects having more severe disease placed greater weight on the importance of expected benefits and less on the risk of toxicity compared to those with mild or no fibrosis.
Conclusions
Whether or not to choose treatment for HCV is a difficult decision for many patients. Treatment is usually recommended for those with moderate to severe liver disease and our results demonstrate that most patients’ preferences are concordant with this practice.
Keywords: Hepatitis C, decision-making, pegylated-interferon, ribavirin
Chronic hepatitis C (HCV) infection is a major healthcare burden among Americans, particularly in American veterans. At present, it is the most common indication for liver transplantation in the country, accounting for 30% of transplant cases (1). It is estimated that the seroprevalence of HCV is 1.8% in the general US population (2) while it is 5.4% in a population of Veterans Affairs medical center users (3).
Although definitive data on long-term outcomes are lacking, studies have shown that sustained virological response (SVR) to treatment appears to be associated with a decreased rate of disease progression and improved survival (4–8). Over the past decade, treatment of HCV has evolved favorably from a 5% to 10% SVR with 24 weeks of interferon monotherapy, to the current 54–56% SVR with 48 weeks of pegylated-interferon and ribavirin (9, 10).
The efficacy of antiviral therapy, however, has to be considered in the context of the natural history of HCV. Cirrhosis develops in 7% to 20% of patients while liver-related mortality occurs in up to 3.7% of patients over an average follow-up of 16 years (11–14). Moreover, chronic HCV does not progress at a uniform rate in all patients. Therefore, although antiviral therapy is effective at reducing progression to cirrhosis and the development of complications related to cirrhosis, at the individual patient level many patients may not derive any benefit from treatment. Furthermore, the medications which are currently available can cause potentially serious adverse effects.
Given the trade-offs involving uncertain benefits and risk of toxicity, treatment for HCV should incorporate patients’ treatment preferences. It is especially important to consider patients’ values in this decision because their valuations of outcomes in HCV differ from physicians’. In contrast to previous studies which have examined patients’ values for multiattribute health states (15, 16) and their preferences for the timing of general health states related to HCV (17), the objective of this study was to ascertain patients’ treatment preferences for HCV in clinical practice at the actual time of decision making.
Methods
Subjects
The study was conducted at two sites: the VA Connecticut Healthcare System Liver Clinic and the Yale University Liver Clinic. The Hepatitis C Resource Center at VA Connecticut is an innovative multidisciplinary model of care that specifically targets veterans with chronic HCV who have psychiatric co-morbidities including mental illness and alcohol or drug abuse. Patients referred to this clinic undergo a pre-treatment intervention that includes, in addition to medical evaluation, a standardized group education class and psychiatric evaluation. Both the VA and University clinics are attended by the same hepatologists.
Because the purpose of this study was to describe patient preferences for treatment at the actual time of decision-making, we recruited consecutive patients eligible for treatment of HCV. Eligibility criteria included chronic HCV, no prior treatment for HCV, known genotype, and liver biopsy within the preceding two years or clinical evidence of cirrhosis. Inclusion criteria were based on the recommendations of the National Institute of Diabetes and Digestive and Kidney Diseases that states that patients with chronic HCV and evidence of chronic hepatitis on liver biopsy, and with no contraindications, should be offered therapy with the combination of peginterferon and ribavirin regardless of the presence of symptoms or genotype (18). Subjects were recruited by their treating physician, advanced practice registered nurse (APRN), or by the research nurse at their liver biopsy appointments. Eligible patients either signed informed consent at their biopsy visit, or when they arrived for their study visit, which was held on the same day as their follow-up appointment with their hepatologist. Subjects who did not have a scheduled liver biopsy were invited to participate by clinic staff, and consented by the research nurse on the day of their study visit.
Data Collection
Data were collected on the day subjects were scheduled to talk to their physician about treatment for HCV. Subjects were asked to come in 90 minutes before their appointment in order to participate in the study. Because expected treatment benefits vary depending on the extent of underlying liver disease, all patients were informed of their biopsy results by their physician or APRN before completing the surveys.
All data were collected in a private room with the help of the research nurse. Before performing the preference task, subjects completed a questionnaire to ascertain sociodemographic characteristics, alcohol and drug use (19–21), social support (22), overall health status (23), HCV-related quality of life (24), mental illness, trust in physician (25) and decisional conflict (26). Decisional conflict was measure using the Decisional Conflict Scale (26), a well-validated instrument composed of 16 items in which scores can range from 0 (representing no decisional conflict) to 100 (representing extremely high decisional conflict). For patients without cirrhosis, we also ascertained subjects’ perceived risk of developing cirrhosis without treatment on a 0 to 100 numeric rating scale (27).
We elicited treatment preferences using Adaptive Conjoint Analysis (ACA, Sawtooth Software ®). Conjoint analysis is a well-validated tool originally developed to understand consumer preferences and predict market shares of innovative products (28–30) and now has been widely used to elicit preferences for health care (31, 32). Conjoint analysis is a decompositional technique that is based on the premise that respondents’ preferences can be calculated based on the value that they attach to the specific attributes of the products under consideration. This method assumes a “composition rule” which states that every attribute level has some value, and that the value of the product is equal to the sum of its part values (33, 34).
We composed an ACA questionnaire to elicit preferences for treatment of HCV with pegylated-interferon and ribavirin. The survey included five treatment characteristics or attributes (benefit, need to monitor blood tests, and risks of flu-like illness, fatigue and depression) and a total of 13 levels which are detailed in Appendix A. In order to ensure that subjects were presented with individualized estimates of potential benefits, we created eight versions of the ACA questionnaire for patients with varying degrees of fibrosis on biopsy (none, mild, moderate or cirrhosis) and genotype (1 or 2) (Detailed in Appendix B). Rates of SVR and outcomes (progression to cirrhosis for patients without cirrhosis and development of liver failure or cancer for patients with cirrhosis) were obtained from the literature (9, 10, 35). We also used patient testimonials obtained from preparatory focus groups to describe potential adverse events (Appendix C) and pictographs to facilitate risk communication.
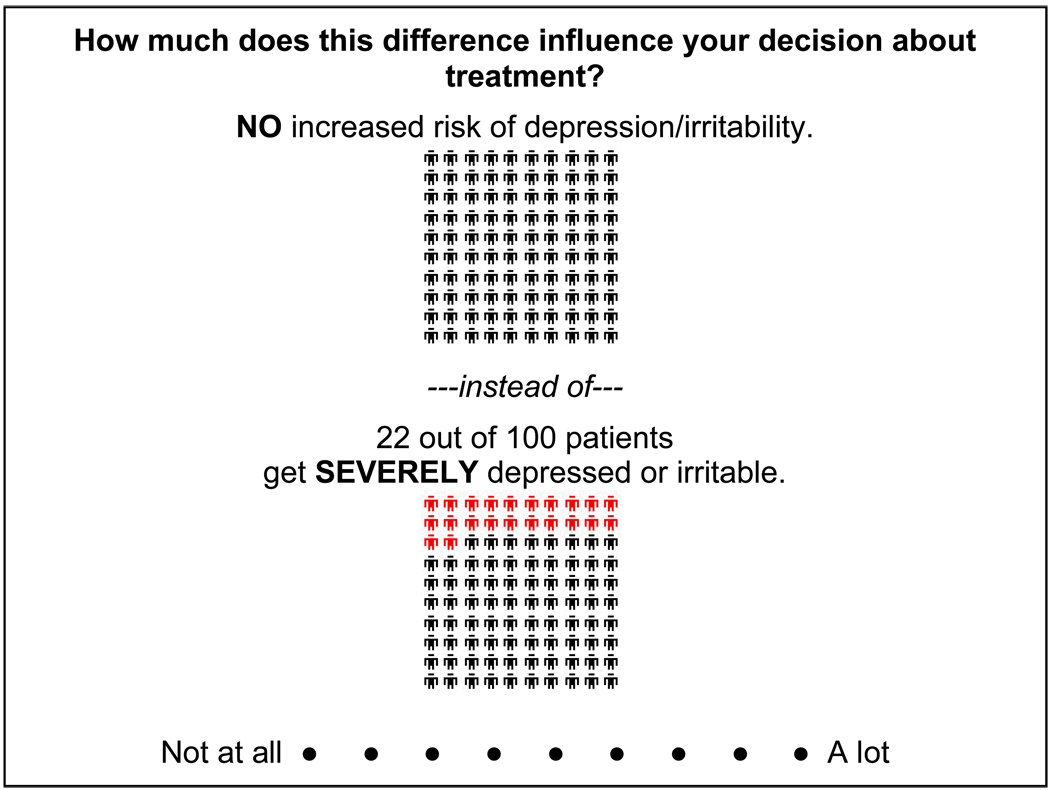
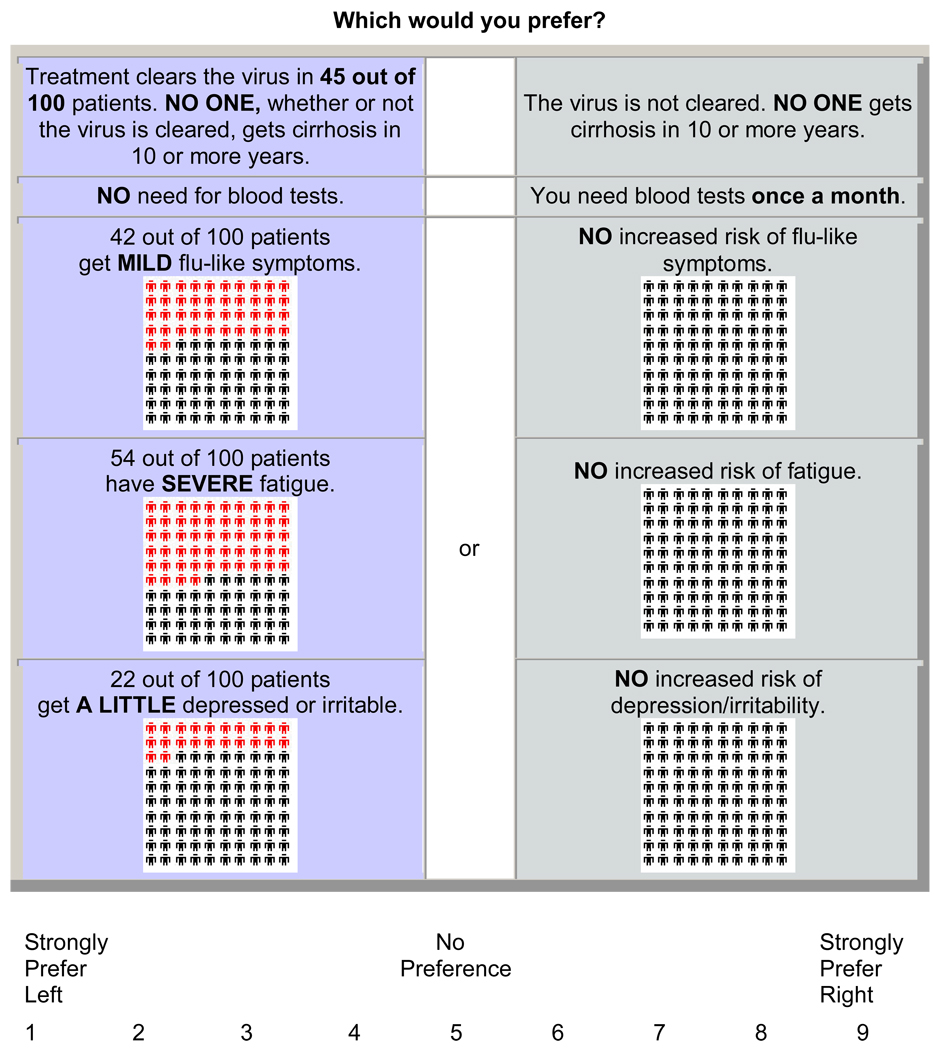
ACA is a hybrid approach of conjoint analysis in that it uses both self-explicated ratings and pairwise comparisons to predict preferences. As such, participants first rated the importance of the difference between best and worst estimates of each characteristic (Figure 1) and subsequently completed a series of paired comparisons (Figure 2). A nine-point scale was used in the pairs section so that finer differences in strength of preference could be derived (36).
Figure 1.
Example of ACA rating task.
Figure 2.
Example of ACA paired comparison task.
The design of the conjoint survey is determined by the ACA Software. ACA ensures that the choices presented in each pair always have different levels of the same attributes, and are displayed at random on either the right or left screen. The minimum number of recommended pairs presented by ACA is determined by a built-in formula 3* (N-n-1)-N, where N is the total number of levels across all attributes and n is the total number of attributes. We included 10 pairs, two more than recommended by the formula [3* (13–5–1)−13 = 8 pairs].
ACA constructs pairs by examining all the possible ways the levels can be combined and then chooses pairs of options for which it expects respondents to be indifferent (based on previous responses). Each question involves choosing one option from a pair in which one is superior in one attribute and the opposing option is superior in the other. If one option is clearly superior to the other based on ACA’s initial estimate of utilities, no additional information is learned. The program uses the information obtained from each paired comparison to update the estimates of each respondent’s utilities and to select the next pair of options. The choice of which levels are presented are based on the three additional premises: 1) that the design should be as “balanced” as possible; 2) that observations should be spread as evenly as possible over all levels, and 3) that the columns of the design matrix should be as orthogonal as possible. Further details describing how pairs are constructed are available (37).
ACA Output
Answers to the survey questions are used by the software to calculate utilities or part worths for each level of each attribute using ordinary least squares regression. The precise methods for how the software computes part worths for the rating and pairs sections have been published (37). In brief, conjoint models are regression models. The coefficients from the model are the utilities. In ACA, regression models are constructed for each individual respondent. The models are constructed based on individual respondent’s ratings of the question included in the survey. This approach assumes that respondent’s ratings reveal some information about how they value the specific characteristics included in the survey. Characteristics which are rated higher are presumed to be of greater value or utility. A respondent’s utility is therefore a measure of their relative preference for each level of each attribute. Utilities are calculated using a least squares updating algorithm. The final utility estimates reflect true least squares (37).
Market simulators are then used to convert the raw utilities into preferences for options specified by the investigator based on the assumption that subjects’ prefer the option with the highest utility. Because we found that patients differed substantially in how they reported being affected by the side effects in preparatory focus groups (38), we examined treatment preferences under two conditions: when treatment was associated with severe side effects and when treatment was associated with mild side effects. We predicted preferences using the randomized first choice model in which utilities are summed across the levels corresponding to each option and then exponentiated and rescaled so that they sum to 100. This model accounts for the error in the point estimates of the part-worths during simulations and is recommended for analysis of individual-level utilities (39). Details related to this model have been previously published (39). Subjects with shares of preferences greater than 50% were classified as preferring therapy.
To determine the relative impact of each attribute included in the ACA survey on subjects’ preferences we divided the range of utilities for each characteristic by the sum of ranges, and multiplying by 100. The relative importances are proportions and sum to 100 (37).
Statistical Analyses
Patient characteristics were entered into SAS computer files (SAS Software, version 9.1, SAS Institute, Inc., Cary, North Carolina). Preference data derived from ACA were imported into and merged with the patient characteristics data set. We examined the association between patient characteristics and treatment preference using the Mann-Whitney U test and chi-square statistic for continuous and categorical variables respectively. We subsequently calculated adjusted odds ratios and 95% confidence intervals using logistic regression. All variables found to be significant at p ≤ 0.05 in bivariate analyses were entered into the model. We used the post-estimation Wald test to assess the individual contribution of each variable. Given possible differences between the VA and University settings, we also performed exploratory subgroup analyses by site.
Results
Patient Characteristics
Of 212 eligible subjects, 178 agreed to participate and 140 performed the ACA task and all completed the entire survey. The computer task was not performed in 38 eligible patients for the following reasons: 21 patients cancelled or did not come to their appointment, six patients did not have the time to complete the task, eight could not participate because of a scheduling error, and the computer malfunctioned on three occasions. The mean (±SD) age of the sample was 51±8, 85% were male, 59% were White and 30% Black. Further details regarding subjects’ characteristics are provided in Table 1.
Table 1.
Subjects’ Characteristics.
| Characteristic | Number (%) Total = 140 | |
|---|---|---|
| Age, years (mean ± SD) | 51 ± 8 | |
| Male | 119 (85) | |
| Hispanic | 19 (14) | |
| Race: White | 82 (59) | |
| Black | 43 (31) | |
| Married | 33 (24) | |
| At least some college education | 63 (45) | |
| Employed | 54 (39) | |
| Veteran | 96 (69) | |
| Excellent or very good overall health status | 30 (21) | |
| HCV-related quality of life* (median, range) | 18 (0–84) | |
| Liver Biopsy: No fibrosis - Genotype 1 | 6 (4) | |
| No fibrosis - Genotype 2 | 0 | |
| Mild fibrosis - Genotype 1 | 43 (31) | |
| Mild fibrosis - Genotype 2 | 9 (6) | |
| Moderate fibrosis - Genotype 1 | 59 (42) | |
| Moderate fibrosis - Genotype 2 | 8 (6) | |
| Cirrhosis - Genotype 1 | 9 (6) | |
| Cirrhosis - Genotype 2 | 6 (4) | |
| History of mental illness | 54 (39) | |
| Alcohol abuse (≥ 5 drinks per day): Never | 50 (36) | |
| Ever | 90 (64) | |
| Drug abuse: Never | 19 (14) | |
| Ever | 121 (86) | |
Possible range 0–100 with larger scores representing worse quality of life.
Treatment Preferences
Sixty-seven percent (N=94) of subjects’ preferred treatment for HCV if associated with mild side effects. The percentage of subjects preferring therapy decreased to 51% (N=72) when it was described as being associated with severe side effects.
The associations of subjects’ characteristics and preference for treatment described as being associated with severe side effects are reported in Table 2a and Table 2b. In unadjusted analyses, we found that women, as well as subjects with a higher perceived risk of developing cirrhosis, more severe liver disease, genotype 2, and worse HCV-related quality of life were more likely to prefer treatment. Those with greater decisional conflict were less likely to prefer treatment. In bivariate analyses, associations with preference for treatment described as having mild side effects were the same as those reported in Table 2a and Table 2b except for genotype 1 versus 2 (66% versus 83%, p=0.1) and gender (65% versus 81%, p=0.1) which were not associated with preference and trust in physician which was [57 (36–68) versus 55 (34–68), p=0.04]. Adjusted analyses are reported in Table 3. We found no other relationships between the remaining demographic characteristics, the use of drugs or alcohol, current health status, or social support and treatment preference.
Table 2.
| Table 2a. Associations between subject characteristics (categorical) and preference for treatment associated with severe side effects | |||
|---|---|---|---|
| Characteristic | Percent Preferring Treatment (N) | P value | |
| Race | White | 50 (41) | 0.7 |
| Other | 53 (31) | ||
| Gender | Male | 48 (57) | 0.05 |
| Female | 71 (15) | ||
| Marital status | Married | 39 (13) | 0.1 |
| Not married | 55 (59) | ||
| Education | Some college | 49 (31) | 0.6 |
| No college | 53 (41) | ||
| Employment status | Employed | 43 (23) | 0.1 |
| Unemployed | 57 (49) | ||
| Site | Veteran | 47 (45) | 0.1 |
| Nonveteran | 61 (27) | ||
| Health status | Excellent/very good | 40 (12) | 0.2 |
| Good/fair/poor | 55 (60) | ||
| Mental illness | No | 48 (41) | 0.4 |
| Yes | 55 (30) | ||
| Degree of fibrosis | Mild/none | 29 (17) | 0.0001 |
| Moderate | 61 (41) | ||
| Severe | 93 (14) | ||
| Genotype | 1 | 49 (54) | 0.03 |
| 2 | 74 (17) | ||
| Alcohol use | Never | 58 (29) | 0.2 |
| Ever | 48 (43) | ||
| Drug abuse | Never | 53 (10) | 0.9 |
| Ever | 51 (62) | ||
| Table 2b. Associations between subject characteristics (continuous) and preference for treatment associated with severe side effects. | |||
|---|---|---|---|
| Median (range) | |||
| Characteristic | Subjects preferring treatment (N=72) | Subjects not preferring treatment (N=68) | P value |
| Age | 52 (23–64) | 53 (26–70) | 0.1 |
| HCV-related quality of life | 28 (0–77) | 14 (0–84) | 0.03 |
| Expectation of developing cirrhosis | 50 (0–100) | 33 (0–100) | <0.0004 |
| Social support | 62 (5–100) | 63 (12–100) | 0.7 |
| Trust in physician | 57 (36–68) | 55 (34–68) | 0.2 |
| Decisional conflict | 25 (0–58) | 34 (0–73) | 0.02 |
Table 3.
Associations* between subject characteristics and preference for HCV treatment associated with severe and mid side effects.
| Characteristic | Preference for treatment associated with severe side effects | Preference for treatment associated with mild side effects |
|---|---|---|
| Degree of fibrosis | 4.6 (2.1 – 10.0) | 2.8 (1.3 – 6.1) |
| Gender | 5.3 (1.3 – 21.1) | 5.3 (1.0 – 28.1) |
| Genotype | 2.9 (0.8 – 10.3) | 2.1 (0.5 – 8.0) |
| HCV-related quality of life | 1.01 ( 0.99 – 1.03) | 1.02 ( 1.00 – 1.04) |
| Expectation of developing cirrhosis | 1.01 (1.00 – 1.03) | 1.01 (0.99 – 1.03) |
| Decisional conflict | 0.97 (0.94 – 0.99) | 0.97 (0.95 – 0.99) |
| Trust | 1.03 (0.97 – 1.08) | 1.05 (0.99 – 1.11) |
Wald 95% confidence interval for adjusted odds ratios
Given that we recruited patients from two distinct sites, we also performed exploratory subgroup analyses by site (Table 4a and Table 4b). In these analyses, patients reporting a history of mental illness were more likely to prefer treatment at the VA (60% versus 37%, p=0.02), but less likely to prefer treatment at the University Clinic (44% versus 71%, p=0.07). In addition, the extent of underlying liver disease was a strong predictor of preference at the VA, but not at the University Clinic (Table 5). At both sites, the majority of patients with either moderate or severe fibrosis preferred treatment. However, subjects at the University Clinic with mild or no fibrosis were more likely to prefer treatment compared to those recruited from the VA (50% versus 24%, p=0.08).
Table 4.
| Table 4a. Associations between subject characteristics (dichotomous) and preferences by site | |||||
|---|---|---|---|---|---|
| VA Clinic Subjects | University Clinic Subjects | ||||
| Characteristic | Percent Preferring Treatment (N= 45) | P value | Percent Preferring Treatment (N = 27) | P value | |
| Race | White | 46 (24) | 0.9 | 57 (17) | 0.3 |
| Other | 48 (21) | 71 (10) | |||
| Gender* | Male | - | - | 50 (12) | 0.09 |
| Female | 75 (15) | ||||
| Marital status | Married | 32 (7) | 0.1 | 55 (6) | 0.6 |
| Not married | 51 (38) | 64 (21) | |||
| Education | Some college | 46 (24) | 0.9 | 64 (7) | 0.9 |
| No college | 48 (21) | 61 (20) | |||
| Employment | Employed | 36 (13) | 0.1 | 56 (10) | 0.5 |
| Unemployed | 53 (32) | 65 (17) | |||
| Alcohol abuse | Never | 52 (15) | 0.5 | 67 (14) | 0.5 |
| Ever | 45 (30) | 56 (13) | |||
| Drug abuse | Never | 33 (4) | 0.3 | 86 (6) | 0.1 |
| Ever | 49 (41) | 57 (21) | |||
| Health status | Excellent/very good | 36 (8) | 0.3 | 50 (4) | 0.5 |
| Good/fair/poor | 50 (37) | 64 (23) | |||
| Mental illness | No | 37 (21) | 0.02 | 71 (20) | 0.07 |
| Yes | 60 (23) | 44 (7) | |||
| Degree of fibrosis | Mild/none | 24 (11) | <0.0001 | 50 (6) | 0.4 |
| Moderate | 61 (25) | 62 (16) | |||
| Severe | 100 (9) | 83 (5) | |||
| Genotype | 1 | 45 (35) | 0.2 | 56 (19) | 0.08 |
| 2 | 64 (9) | 89 (8) | |||
| Table 4b. Associations between subject characteristics (continuous) and preferences by site. | ||||||
|---|---|---|---|---|---|---|
| Median (range) VA Clinic Subjects | Median (range)University Clinic Subjects | |||||
| Characteristic | Preferring treatment (N=45) | Not preferring treatment (N=51) | P value | Preferring treatment (N=27) | Not preferring treatment (N=17) | P value |
| Age | 53 (39–64) | 54 (34–70) | 0.9 | 46 (23–64) | 51 (26–69) | 0.1 |
| HCV-related quality of life | 27 (90–70) | 11 (0–80) | 0.08 | 30 (2–77) | 18 (0–84) | 0.5 |
| Expectation of developing cirrhosis | 50 (0–100) | 20 (0–85) | 0.0002 | 50 (0–100) | 50 (0–100) | 0.8 |
| Social support | 53 (5–98) | 62 (12–99) | 0.1 | 84 (32–100) | 67 (29–100) | 0.2 |
| Trust in physician | 66 (36–75) | 64 (45–79) | 0.2 | 66 (48–77) | 64 (50–77) | 0.7 |
| Decisional conflict | 25 (0–50) | 33 (90–73) | 0.1 | 29 (0–58) | 42 (12–71) | 0.03 |
Unable to test the effect of gender at the VA.
Table 5.
Percent of subjects choosing treatment by severity of liver disease by site
| Site | No/Mild Fibrosis (N) | Moderate Fibrosis (N) | Severe Fibrosis (N) | P Value |
|---|---|---|---|---|
| VA | 24% (11) | 61% (25) | 100% (9) | <0.0001 |
| University Clinic | 50% (6) | 61% (16) | 83% (5) | <0.4 |
Relative Impact of Treatment Characteristics
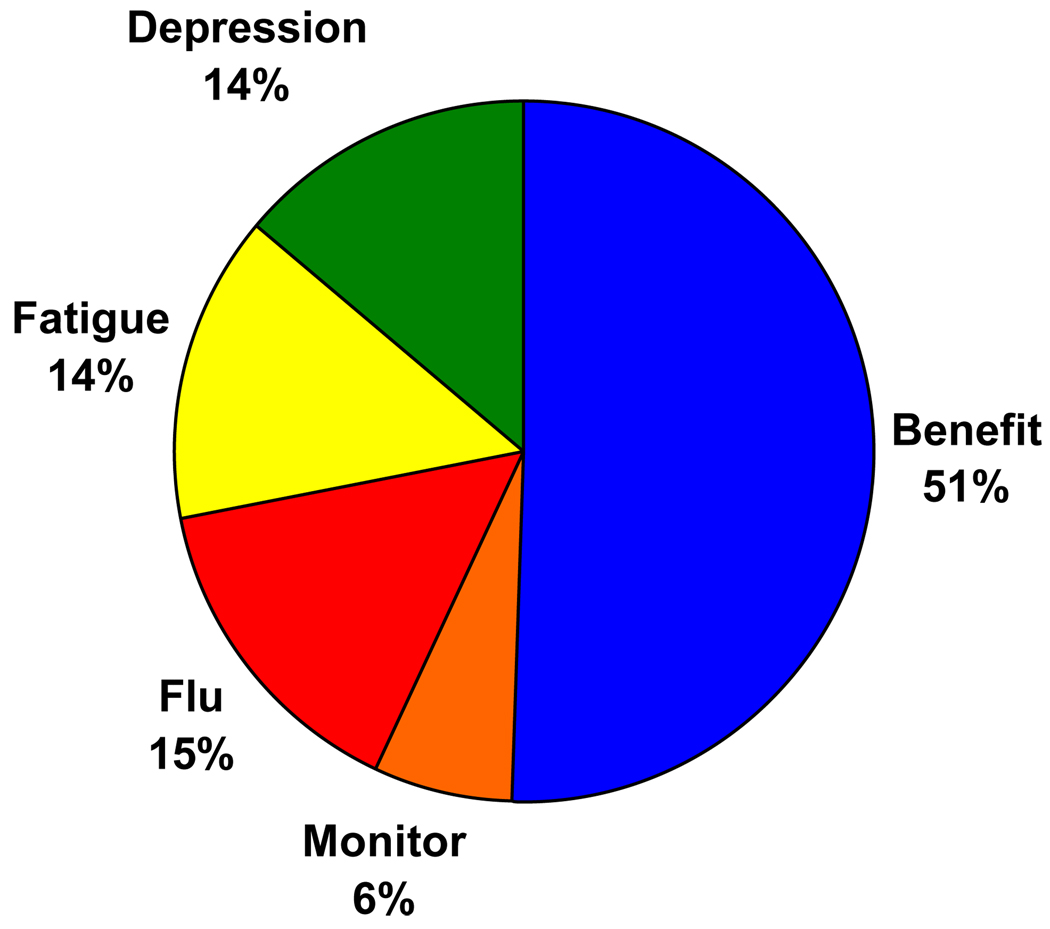
The relative impact of the treatment characteristics included in the survey on subjects’ preferences is illustrated in Figure 3. Overall, the likelihood of benefit was most important to subjects and the need for blood test monitoring least important. The risk of fatigue, depression and flu-like illness all had similar influences on subjects’ preferences. Table 6 displays the impact of each characteristic by severity of liver disease. These results demonstrate significant associations between the severity of liver disease on biopsy and the relative impact of risk and benefits, with subjects having more severe disease placing greater weight on the importance of expected benefits and less on the risk of toxicity compared to those with mild or no fibrosis. This pattern was also observed in subgroup analyses by site.
Figure 3.
Relative impact of treatment characteristics included in the ACA survey on subjects’ preferences.
Table 6.
Relative impact of treatment characteristics on subjects’ preferences by severity of liver disease
| Relative Impact** |
|||
|---|---|---|---|
| Characteristic | Mild/No Fibrosis | Moderate Fibrosis | Severe Fibrosis |
| Benefit | 33 ± 24 | 51 ± 25* | 64 ± 16* |
| Monitor | 7 ± 5 | 8 ± 6 | 8 ± 7 |
| Flu | 20 ± 11 | 13 ± 11* | 10 ± 7* |
| Fatigue | 21 ± 12 | 13 ± 10* | 9 ± 7* |
| Depression | 19 ± 11 | 15 ± 15 | 9 ± 8* |
p<0.05 compared to Mild/None (least square means adjusted for multiple comparisons using Tukey’s procedure).
The relative impact reflect the impact that each characteristic has on subjects’ preferences. The values are proportions that sum to 100.
Discussion
In summary, we found significant variability in preferences for treatment of HCV amongst patients with mild to moderate disease. In contrast, almost all patients with more severe fibrosis had a strong preference for treatment. To the best of our knowledge this is the first study to examine treatment preferences for HCV, using individualized outcome data, in routine clinical practice at the time of decision making. Our results mirror current recommendations from the National Institutes of Health Consensus Development Conference Panel (40), as well as guidelines from the American Gastroenterological Association and (41) American Association for the Study of Liver Diseases (42), which recommend treatment for all eligible patients with moderate to severe liver disease and individualized treatment planning based on patient preferences for those with milder degrees of inflammation on liver biopsy.
Other studies have also demonstrated significant variability in treatment preferences. Falck-Ytter et al (43) described lower rates of refusal, but included populations with patients who were not eligible for treatment. Khokhar et al (44) found that 41% of eligible patients refused or deferred treatment. However, the impact of underlying liver disease on preferences was not evaluated in this paper. The most common reason for deferral in Khokhar et al’s study was the lack of symptoms experienced by patients and their concerns related to side effects (44). In this study we found similar concerns related to toxicity, and further noted that the impact of toxicity varies with the severity of underlying liver disease. However, the finding that the extent of underlying liver disease is a stronger predictor of preference than HCV-related quality of life suggests that it is patients’ worry related to their prognosis, and not symptom burden that is the primary determinant of decision making. We also found that subjects with greater decisional conflict were more likely to refuse treatment. This finding is consistent with studies demonstrating that people are more likely to opt out or defer decision making when faced with a difficult choice task (45) and emphasize the need to provide patients with adequate decisional support.
We did not find any effect of age or race on patient preferences. These results suggest that reasons outside of patient preferences account for the lower frequency of treatment observed among women, older adults, and non-white patients found in previous studies (46, 47). Exploratory subgroup analyses by site revealed several interesting findings. First, treatment preferences amongst subjects recruited from the VA were strongly related to severity of underlying liver disease and perceived risk of cirrhosis where as this association was much weaker amongst subjects cared for at the University Clinic. This difference may be due to the formal education classes that most veterans with HCV attend. An alternative explanation may be that subjects outside of the VA may have stronger opinions regarding therapy before consulting with a specialist compared to the veteran population. In addition, we found that subjects who reported having a history of mental illness were more likely to prefer therapy at the VA, while they were less likely to prefer therapy in the University Clinic. This difference is almost certainly due to the specialized care afforded to patients with co-morbidities at the Hepatitis C Resource Centers developed within the VA healthcare system.
The strengths of this study lie in the methods used to evaluate preferences as well as the successful recruitment of a substantial number of patients at the time of decision making. There are also several limitations of this study. Although there are no clear methods for determining sample size in ACA studies, the total population recruited for this study is in keeping with previous analyses using similar methods (28, 48, 49). We present the subgroup analyses because of probable differences between University-based and VA populations; however, it is important to note that only a small number of patients were able to be recruited from the University-based clinic. The subgroup results should be viewed as hypotheses generating only.
Patients with HCV cover diverse sociodemographic groups and participants recruited for this study may not be representative of other community-based samples. In addition, because we were interested in describing patient preferences at the actual time of decision making, only patients eligible for treatment were recruited. Preference data from the general population would be better suited to inform public health and/or policy decisions. Lastly, the relative impact of the medication characteristics on patients’ preferences refer only to the attributes (and the ranges of their respective levels) included in the ACA survey and do not account for other concerns which may be important determinants of patients’ decision making.
Treatment planning may be relatively straightforward for patients with significant fibrosis given the concordance between guideline recommendations and patients’ strong preference for treatment. Decision support is most important for patients with milder disease in whom there is substantial variability in treatment preferences due to the differences in how patients value the potential risks and uncertain benefits associated with therapy.
Acknowledgements
We very much appreciate the support of Carol Eggers, APRN and Martha Shea, RN who greatly facilitated recruitment for this study.
This study was funded by the VA Health Services Research Department Grant IIR 03–621–1 and the Yale Liver Center Pilot Project Grant DK P30 34989. Dr. Fraenkel is also supported by the K23 Award AR048826-01 A1.
Appendix A Medication Attributes and Levels
| Attributes | Levels |
|---|---|
| Benefit |
|
| Monitoring blood tests for side effects |
|
| Flu-like illness |
|
| Fatigue |
|
| Depression/irritability |
|
Modified depending on biopsy results.
Appendix B Individualized benefits for specified degrees of liver disease and genotype
| No fibrosis - Genotype 1 | Treated: The virus is cleared in 45 out of 100 patients. No one, whether or not the virus is cleared, has cirrhosis after 10 or more years. |
| Not treated: The virus is not cleared; No one has cirrhosis after 10 or more years. | |
| No fibrosis - Genotype 2 | Treated: The virus is cleared in 80 out of 100 patients. No one, whether or not the virus is cleared, has cirrhosis after 10 or more years. |
| Not treated: The virus is not cleared; No one has cirrhosis after 10 or more years. | |
| Mild fibrosis - Genotype 1 | Treated: The virus is cleared in 45 out of 100 patients. None of these patients have cirrhosis after 10 or more years. |
| Not treated: The virus is not cleared; 10 out of 100 people have cirrhosis after 10 or more years. | |
| Mild fibrosis - Genotype 2 | Treated: The virus is cleared in 80 out of 100 patients. None of these patients have cirrhosis after 10 or more years. |
| Not treated: The virus is not cleared; 10 out of 100 people have cirrhosis after 10 or more years. | |
| Moderate fibrosis - Genotype 1 | Treated: The virus is cleared in 45 out of 100 patients. None of these patients have cirrhosis after 10 or more years. |
| Not treated: The virus is not cleared; 50 out of 100 people have cirrhosis after 10 or more years. | |
| Moderate fibrosis - Genotype 2 | Treated: The virus is cleared in 80 out of 100 patients. None of these patients have cirrhosis after 10 or more years. |
| Not treated: The virus is not cleared; 50 out of 100 people have cirrhosis after 10 or more years. | |
| Cirrhosis - Genotype 1 | Treated: The virus is cleared in 30 out of 100 patients. The risk of having liver failure and liver cancer is MUCH lower in these patients. |
| Not treated: The virus is not cleared. In 10 years, 40 out of 100 patients have liver failure and 7 out of 100 patients have liver cancer. | |
| Cirrhosis - Genotype 2 | Treated: The virus is cleared in 70 out of 100 patients. The risk of having liver failure and liver cancer is MUCH lower in these patients. |
| Not treated: The virus is not cleared. In 10 years, 40 out of 100 patients have liver failure and 7 out of 100 patients have liver cancer. |
Appendix C Patient testimonials used to illustrate side effects
Flu-like illness
Mild: You may have mild aches, fever or chills, but it does not interfere with your activities, such as working or caring for children.
“Like having a runny nose, hot and cold chills, and just feeling stiff. My voice would start to change and then the aches would start to come in. Sometimes you just have this ache through the middle of your bones.”
Severe: You may have severe body aches, joint pain, fever and chills, nausea, vomiting and diarrhea, and it interferes a lot with your activities. You may have to take many days off from work, or not be able to take care of your children at times.
“Throw up, diarrhea, almost on a daily basis. Even the thought of it right now, I just get sick thinking about it. It made my joints hurt. Aches and pains and God only knows how much Tylenol and Aleve I took during that time. I’d do my injection. Within an hour I’d be wrapped up in an electric blanket freezing, soaking with sweat. It was brutal.”
Fatigue
Mild: You may feel more tired than usual, but it does not interfere with your important activities, such as working or caring for children.
“I used to look forward to getting home and taking a nap. Sometimes you get up in the morning and you feel like you are hung over. It’s a chore to go to the grocery store. It’s a chore to get my laundry done.
Severe: You may feel extremely tired a lot of the time, and it interferes a lot with your activities. You may have to take many days off from work, or not be able to take care of your children at times.
“I was tired. I couldn’t deal with anything. When I’d go to work I just wanted to go to sleep. But I made it through lunch. I’d sleep for an hour. I wouldn’t bother eating, because I’d just fall asleep. I would get home and sometimes I couldn’t even get in the shower and I would just take my clothes off and on the bed, boom, and I’d be out.”
Depression/irritability
Mild: You may feel very sad or irritable more often than usual, but it does not interfere with your important activities, such as working. You may have some trouble getting along with family, friends and co-workers.
“The emotional part is being on a roller coaster. It was down, up, down, up. I just wanted to be left alone. I was snapping. You know my fuse was definitely shortened. I’d say all of the things you are only meant to be thinking.”
Severe: You may feel very depressed and irritable and be worried about losing your temper. You may feel like you want to isolate yourself from others. You may have a lot of trouble getting along with your family, friends and co-workers. You will need to see a doctor or therapist to help you.
“I cried, and cried and cried. Uncontrollable tears for two months. My mood swings were wild. I was in a dark place. I just felt awful. It was like an uncontrollable sense of anger. I have children and they would be in absolute avoidance of me. If someone pushed my button wrong, I exploded. I’m surprised I have any friends left.”
Management of Side Effects
The experience of this clinic staff is that these side effects can be managed in most people.
Often the side effects are worse in the first few months.
For flu symptoms, Tylenol and “drink a lot of water” are the main things recommended.
For fatigue, there is no treatment, but the clinic can help you find ways to manage your responsibilities.
For depression or irritability, you can be given medications and be followed by psychiatric staff.
All these things may be helpful to many people, but do not help everyone.
References
- 1.Pessoa MG, Wright TL. Hepatitis C infection in transplantation. Clin Liv Dis. 1997;1:663–690. doi: 10.1016/s1089-3261(05)70328-7. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 3.Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88–96. doi: 10.1002/hep.20502. [DOI] [PubMed] [Google Scholar]
- 4.Omata M, Shiratori Y. Long-term effects of interferon therapy on histology and development of hepatocellular carcinoma in hepatitis C. J Gastroen Hepatol. 2000;15:E134–E140. doi: 10.1046/j.1440-1746.2000.02115.x. [DOI] [PubMed] [Google Scholar]
- 5.Shindo M, Hamada K, Oda Y, Okuno T. Long-term follow-up study of sustained biochemical responders with interferon therapy. Hepatology. 2001;33:1299–1302. doi: 10.1053/jhep.2001.24100. [DOI] [PubMed] [Google Scholar]
- 6.Kasahara A, Hayashi N, Mochizuki K, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Hepatology. 1998;27:1394–1402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123:483–491. doi: 10.1053/gast.2002.34785. [DOI] [PubMed] [Google Scholar]
- 8.Bruno S, Stroffolini T, Colombo M, et al. Sustained Virological Response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 11.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 12.Di Bisceglie AM, Goodman ZD, Ishak KG, Hoofnagle JH, Melpolder JJ, Alter HJ. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–974. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 13.Koretz RL, Abbey H, Coleman E, Gitnick G. Non-A, non-B post-transfusion hepatitis. Looking back in the second decade. Ann Intern Med. 1993;119:110–115. doi: 10.7326/0003-4819-119-2-199307150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Tremolada F, Casarin C, Alberti A, et al. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol. 1992;16:273–281. doi: 10.1016/s0168-8278(05)80657-9. [DOI] [PubMed] [Google Scholar]
- 15.Cotler SJ, Patil R, McNUtt RA, et al. Patients' values for health states associated with hepatitis C and physicians' estimates of those values. Am J Gastroenterol. 2001;96:2730–2736. doi: 10.1111/j.1572-0241.2001.04132.x. [DOI] [PubMed] [Google Scholar]
- 16.Schackman BR, Teixeira PA, Weitzman G, Mushlin AI, Jacobson IM. Quality-of-life tradeoffs for hepatitis C treatment: Do patients and providers agree? Med Decis Making. 2008;28:233–242. doi: 10.1177/0272989X07311753. [DOI] [PubMed] [Google Scholar]
- 17.Treadwell JR, Kearney D, Davila M. Health Profile Preferences of Hepatitis C Patients. Dig Dis Sci. 2000;45:345–350. doi: 10.1023/a:1005420828332. [DOI] [PubMed] [Google Scholar]
- 18.Chronic Hepatitis C: Current Disease Management. 2008 Dec; http://digestive.niddk.nih.gov/ddiseases/pubs/chronichepc/#g.
- 19.Buchsbaum DG, Buchanan RG, Centor RM, et al. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med. 1991;115:774–777. doi: 10.7326/0003-4819-115-10-774. [DOI] [PubMed] [Google Scholar]
- 20.Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 21.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism instrument. Am J Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 22.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Snow KK, Kosinski M, Gandek B. Boston: The Health Institute, New England Medical Center; 1993. SF-36 health status survey manual. [Google Scholar]
- 24.Bayliss MA, Gandek B, Bungay KM, Sugano D, Hsu MA, Ware JE. A questionnaire to assess the generic and disease specific health outcomes of patients with chronic hepatitis C. Qual Life Res. 1998;7:39–55. doi: 10.1023/a:1008884805251. [DOI] [PubMed] [Google Scholar]
- 25.Kao AC, Green DC, Zaslavsky AM, Koplan JP, Cleary PD. The relationship between method of physician payment and patient trust. JAMA. 1998;280:1708–1714. doi: 10.1001/jama.280.19.1708. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 27.Stacey D, O'Connor AM, DeGrasse C, Verma S. Development and evaluation of a breast cancer prevention decision aid for higher-risk women. Health Expectations. 2003;6:3–18. doi: 10.1046/j.1369-6513.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraenkel L, Bogardus ST, Wittink DR. Understanding patient preferences for the treatment of lupus nephritis using adaptive conjoint analysis. Med Care. 2001;39:1203–1216. doi: 10.1097/00005650-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Green PE, Srinivasan V. Conjoint analysis in marketing: new developments with implications for research and practice. J Marketing. 1990;54:3–17. [Google Scholar]
- 30.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan M, Scott DA, Reeves C, et al. Eliciting public preferences for healthcare: a systematic review of techniques. Health Technology Assessment (Winchester, England) 2001;5:1–186. doi: 10.3310/hta5050. [DOI] [PubMed] [Google Scholar]
- 32.Bridges JFP. Stated preference methods in health care evaluation: an emerging methodological paradigm in health economics. Appl Health Econ Health Pol. 2003;2:213–224. [PubMed] [Google Scholar]
- 33.Green PE, Rao VR. Conjoint measurement for quantifying judgemental data. J Market Res. 1971;8:355–363. [Google Scholar]
- 34.Green PE, Srinivasan V. Conjoint analysis in consumer research: issue and outlook. J Consumer Res. 1978;5:102–123. [Google Scholar]
- 35.Hadziyannis SJ, Sette HJ, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 36.Johnson RM. Adaptive Conjoint Analysis. Sawtooth Software Conference Proceedings. 1987:253–265. [Google Scholar]
- 37.ACA technical paper. 2008 Dec; http://www.sawtoothsoftware.com/download/techpap/acatech.pdf.
- 38.Fraenkel L, McGraw S, Wongcharatrawee S, G-T G. Patients’ experiences related to anti-viral treatment for hepatitis C. Pt Educ Counsel. 2006;62:148–155. doi: 10.1016/j.pec.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Advanced Simulation Module for Product Optimization. 2008 Dec; http://www.sawtoothsoftware.com/download/techpap/asmtech.pdf.
- 40.National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed]
- 41.American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 43.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136:288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 44.Khokhar O, Lewis J. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis and Sci. 2007;52:1168–1176. doi: 10.1007/s10620-006-9579-1. [DOI] [PubMed] [Google Scholar]
- 45.Dhar R. Context and task effects on choice deferral. Marketing Letters. 1997;8:119–130. [Google Scholar]
- 46.Butt AA, Wagener M, Shakil AO, Ahmad J. Reasons for non-treatment of hepatitis C in veterans in care. J Viral Hepatitis. 2005;12:81–85. doi: 10.1111/j.1365-2893.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 47.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. J Gen Intern Med. 2005;20:754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Schaik G, Dijkhuizen AA, Huirne RBM, Benedictus G. Adaptive conjoint analysis to determine perceived risk factors of farmers, veterinarians and AI technicians for introduction of BHV1 to dairy farms. Prev Vet Med. 1998;37:101–112. doi: 10.1016/s0167-5877(98)00102-0. [DOI] [PubMed] [Google Scholar]
- 49.Beusterien KM, Dziekan K, Flood E, Harding G, Jordan JC. Understanding patient preferences for HIV medications using adaptive conjoint analysis: Feasibility assessment. Value Health. 2005;8:453–461. doi: 10.1111/j.1524-4733.2005.00036.x. [DOI] [PubMed] [Google Scholar]