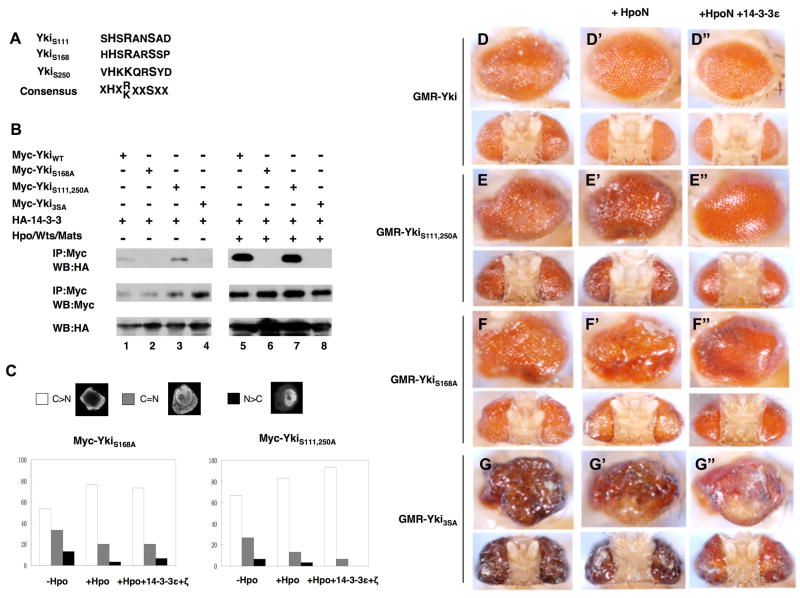
Figure 6. Phosphorylation at S111 and S250 regulates Yki independent of 14-3-3.
(A) The alignment of Yki sequences surrounding S111, S168 or S250 with the consensus sequence for Wts phosphorylation site indicated underneath. X: any amino acid.
(B) Mutating S111 and S250 does not affect 14-3-3 binding but mutating S168 abolishes 14-3-3 binding. S2 cells were transfected with indicated Myc-tagged wild type and mutant Yki constructs and HA-14-3-3 constructs with or without Hpo/Wts/Mats coexpression, followed by immunoprecipitation and western blot analyses with indicated antibodies.
(C) 14-3-3 regulates nuclear localization of YkiS111, 250A but not YkiS168A. S2 cells were transfected with HA-SD, Myc-YkiS111, 250A or Myc-YkiS168A, without or with coexpression of Hpo or Hpo plus 14-3-3. Cells were immunostained with Myc and HA antibodies, and the subcellular localization of Myc-YkiS111, 250A or Myc-YkiS168A was monitored using confocal microscopy. Cells with different nucleocytoplasmic distributions of Myc tagged Yki were counted. A total of 100 cells were counted for each Yki construct. C>N: cells contain higher levels of Yki in the cytoplasm than in the nucleus. C=N: cells contain Yki equally distributed in cytoplasm and nucleus. N>C: cells contain higher levels of Yki in the nucleus than in the cytoplasm. The y axis indicates the percentage of cells in each category.
(D-G″) Side (top) or (bottom) dorsal views of adult eyes expressing GMR-Gal4 UAS-Yki (D), GMR-Gal4 UAS-YkiS111, 250A (E), GMR-Gal4 UAS-YkiS168A (F) or GMR-Gal4 UAS-Yki3SA (G). HpoN (D′-G′) or HpoN and 14-3-3 (D″-G″) were coexpressed with the wild type or mutant Yki as indicated.