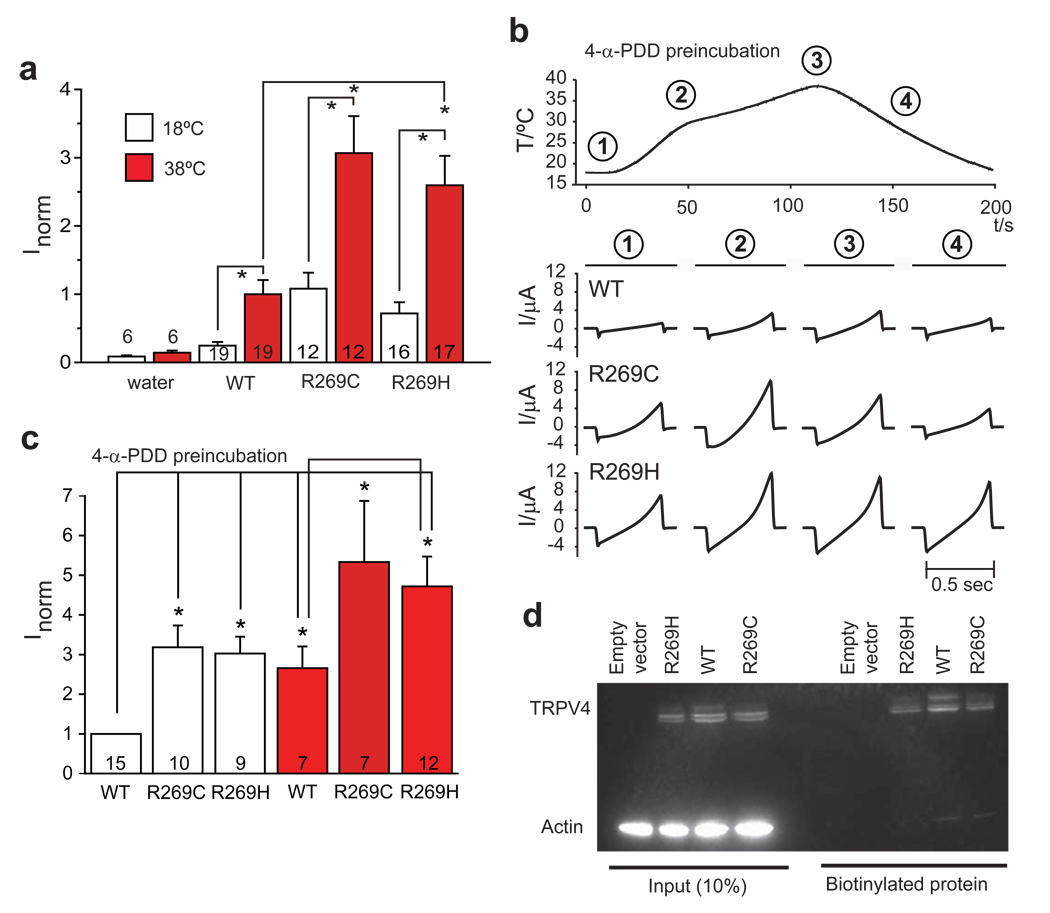
Figure 3.
The R269C and R269H mutations cause increased TRPV4 currents without a change in membrane localization. a) WT and mutant TRPV4 were expressed in Xenopus oocytes and currents were measured at 18°C and after heating to 38°C. Currents obtained at −100 mV were normalized to currents in WT at 38°C. Basal and stimulated channel activities of both mutants were significantly increased compared to WT, p<0.006. Data are averaged from the number of experiments per condition shown in the columns; error bars, s.e.m. *p<0.006. b) Representative current ramps obtained after pre-incubation in 5 µM 4-α-PDD (obtained from clamping cells from −100 to +100 mV in 500 ms). c) Summary of the data obtained in b). Currents obtained at −100 mV were normalized to WT at 18°C. *p<0.05. d) Immunodetection of TRPV4 proteins shows appropriately sized bands for TRPV4 in 10% of whole cell extract inputs and the biotinylated fraction. There is no difference in the amount of WT compared to mutant TRPV4 in the membrane fraction (biotinylated fraction). Shown is one representative blot of four independent experiments.