Table 1.
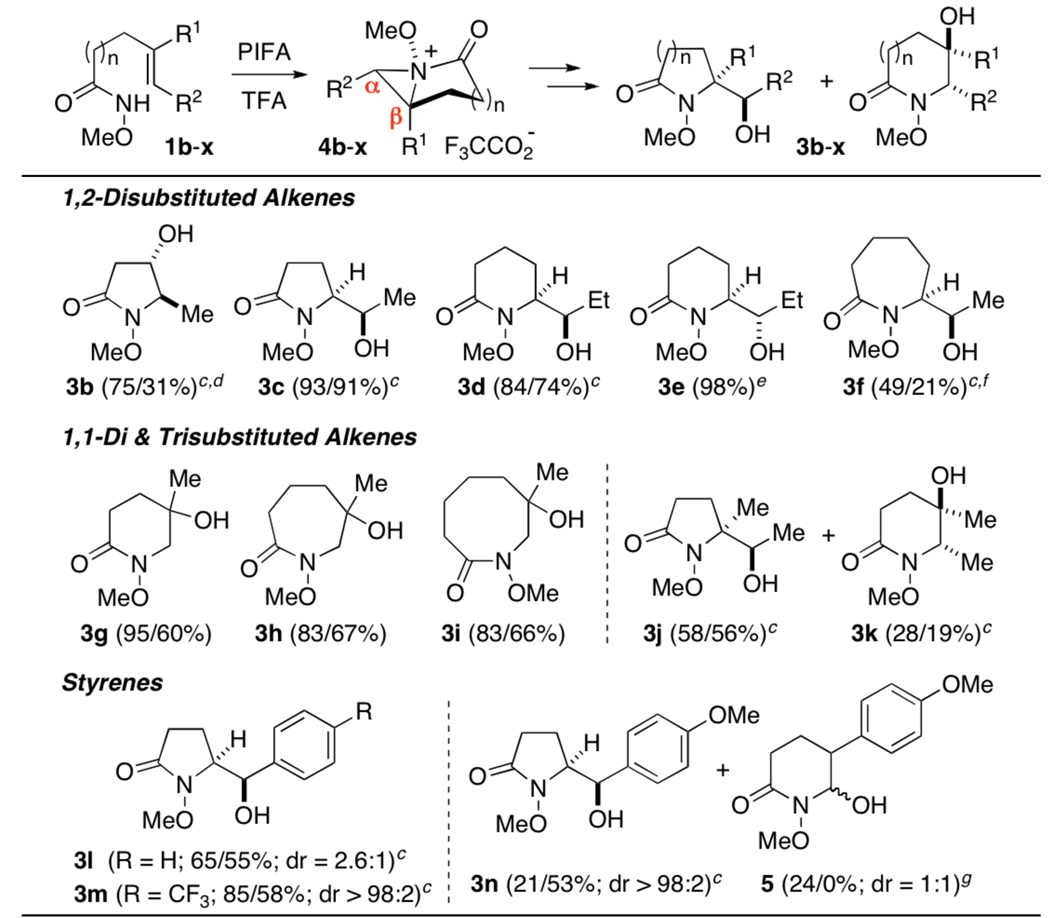 |
Conditions: 1, PIFA (1.2 equiv), TFA (1.0/0.0 equiv), CH2Cl2 (0.15 M), 0 °C; then NH3-MeOH, 20 min.
Isolated yields of oxamidations conducted with and w/o TFA, after purification by flash chromatography.
Generated from E-1.
Workup via hydrazinolysis.
Generated from Z-1.
An azocan-2-one, resulting from β-opening, was also isolated as a single diastereomer in 18/10% yield.
See supporting information for the possible origins of rearrangement product 5.
