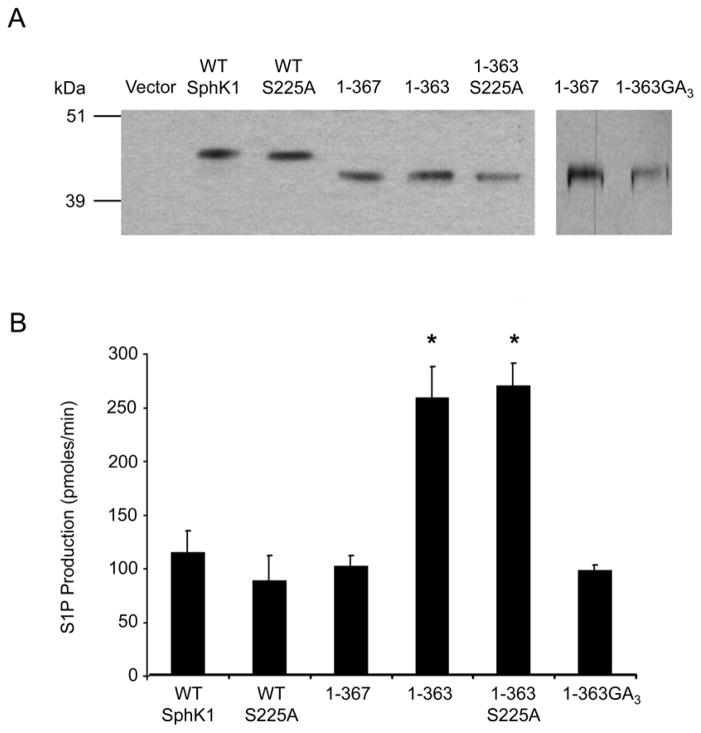
Figure 2. Examination of the 364–367 region and the effect of ERK1/2 phosphorylation on the catalytic activity of the 1–363 mutant.
(A) Truncation and site-directed mutants of SphK1 were stably transfected into HEK293 cells, partially affinity purified and the relative expression levels of the SphK1 proteins were determined by Western blot analysis and densitometric analysis using Image J. Representative Western blots of the normalized protein expression are shown. The 1-363GA3 protein is from a separate blot with 1–367 as a loading control. (B) In vitro SphK1 activity assays were performed in triplicate using equal quantities of partially affinity purified protein determined by densitometric analysis. SphK1 activity is expressed as pmoles of S1P formed per min. (n=4, * p<0.001)