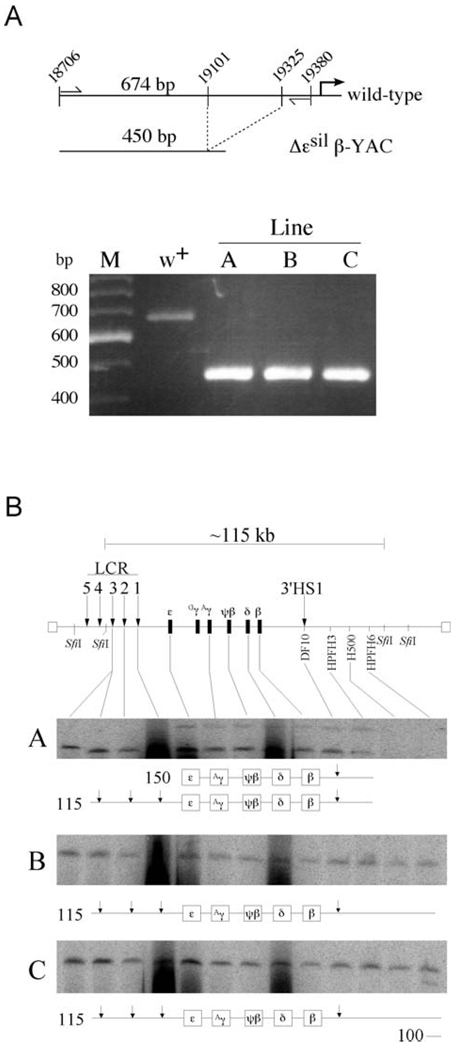
Figure 2.
Confirmation of the ε-silencer deletion by PCR analysis and structural analysis of Δεsil β-YAC transgenic lines. (A) PCR analysis followed by gel electrophoresis to detect the ε-globin silencer deletion. A map of the wild-type region showing the location of the primers (thin vertical arrows) used to amplify the distal ε-globin promoter region. The wild-type ε-globin sequence amplified a 674-bp fragment, and the mutant ε-globin promoter amplifies a 450-bp fragment. As seen in the agarose gel stained with ethidium bromide, the PCR amplification in the wild-type lane showed a single fragment of 674 bp in length, while a 450-bp fragment was PCR amplified from each of the Δεsil β-YAC transgenic lines. M is a 100-bp marker with the fragment lengths labeled to the left of the picture of the agarose gel. (B) We used pulsed-field gel electrophoresis followed by Southern blot hybridization analysis to determine the structural integrity of the integrated β-YAC transgene. At the top of the panel is a schematic diagram of the β-globin locus that includes the LCR (vertical arrow), the globin gene cluster, and 3′HS1. Above the locus is a depiction of the 115-kb SfiI fragment encompassing most of the β-globin locus from 5′HS3 to the breakpoint of HPFH6 approximately 53 kb downstream of the β-globin gene. The probes used to determine the relative intactness of the β-YACs are labeled along the locus and are listed in Experimental Procedures. The resulting autoradiograms of transgenic lines A, B, and C are shown; note that each line contains at least one intact β-globin locus. A representation of the structures of the SfiI fragments is drawn below each autoradiogram. The first lane in each autoradiogram contains DNA from a mouse erythroleukemia cell line containing a single intact β-YAC, as determined by structural analysis and fluorescent in situ hybridization, used as a control (27).