Abstract
The highly toxic oxyanion tellurite has to enter the cytoplasm of microbial cells in order to fully express its toxicity. Here we show that in the phototroph Rhodobacter capsulatus, tellurite exploits acetate permease (ActP) to get into the cytoplasm and that the levels of resistance and uptake are linked.
Tellurite exerts its toxic action in the cytoplasmic compartment mostly by triggering an increase in the generation of reactive oxygen species (ROS) (4, 5, 10). This implies that the acquisition of the oxyanion by the cell is a prerequisite for the full development of its toxicity and suggests, as a consequence, that diminished uptake would result in increased resistance.
The link between tellurite uptake and toxicity in bacterial cells has been previously discussed in a limited number of reports (2, 3, 7). In Escherichia coli, it was shown that mutations in a phosphate transport system confer a high level of resistance to tellurite and that the oxyanion competes very efficiently for 32Pi transport (7). In the Gram-positive bacterium Lactococcus lactis, mutations associated with proteins PstA and PstD (phosphate transport), but also those associated with protein ChoQ (proline/glycine-betaine/carnitine/choline transport), were shown to confer a higher level of resistance relative to the wild type (8). Finally, in the phototrophic species Rhodobacter capsulatus it was suggested that a phosphate transporter might also be responsible for tellurite uptake (3). This hypothesis has recently been questioned because of the extremely high concentration of Pi needed to significantly inhibit tellurite uptake (2). Conversely, acetate was found to decrease the uptake of tellurite, when present at concentrations as low as 1 μM (2). This finding, along with the fact that lactate and pyruvate, but not malate and succinate, act similarly as competitors of tellurite uptake, led to the proposal that, in R. capsulatus, the oxyanion enters the cell via a monocarboxylate transport system (2).
In this work, we show that an acetate transport system is utilized by tellurite to enter R. capsulatus cells and that limitation of this uptake drastically increases the resistance to the oxyanion.
Mutant isolation.
Experiments of prolonged (15 days) incubation of R. capsulatus B100, in RCV minimal medium (9) in the presence of TeO32−, gave a number of resistant clones. A few of the resulting colonies showed, in the presence of tellurite, a dark brown pigmentation clearly different from the typical black color developed by most of the resistant mutants (Fig. 1). This finding suggested that the paler pigmentation of those mutants could be due to a decreased accumulation of reduced elemental tellurium in the cytoplasm, possibly as a consequence of a lower, or abolished, tellurite uptake. One of the mutants, RTE36, was chosen for further studies. Analysis of the mutant showed that, indeed, it was able to grow aerobically in the presence of tellurite at concentrations that completely blocked the growth of the wild type and that the uptake of the oxyanion was decreased to approximately 20% of that of the wild type (Table 1).
FIG. 1.
Wild-type B100 (B100-wt) and tellurite-resistant (Ter) mutants RTE14 and RTE36 grown on minimal medium plates with 0 and 25 μM TeO32−.
TABLE 1.
Resistance to and uptake of tellurite in the mutant strain carrying the plasmids used in this work
| Strain | Tellurite resistance (OD660)a | Tellurite uptake (% of wild type)b |
|---|---|---|
| B100-5 | NG | 100 |
| RTE36-5 | 1.126 ± 0.047 | 22.1 ± 3.3 |
| RTE36-32 (actP) | NG | 113.7 ± 11.7 |
| RTE36-40 (acs + actP) | NG | 142.84 ± 23.6 |
| RTE36-42 (acs) | 1.083 ± 0.018 | 19.08 ± 1.34 |
| RTE36-320 (actP) | NG | 126.0 ± 11.6 |
Resistance was measured as optical density at 660 nm (OD660) reached by liquid cultures after 48 h, in the presence of 50 μM TeO32−. NG, no growth.
Tellurite uptake was measured in the presence of 100 μM TeO32−. The wild-type uptake rate, corresponding to 100%, is 4.274 ± 0.203 nmol/mg protein/min.
Complementation of the mutant.
The mutant RTE36 was conjugated with an R. capsulatus cosmid library (1) in an attempt to isolate DNA fragments able to complement the resistance phenotype (i.e., to restore the sensitivity to tellurite). Two complementing cosmids, pRB31 and pRB32, were isolated and shown, on plating, to partially restore the sensitivity to the oxyanion (not shown). The complementing cosmid pRB32 was further tested and proved to decrease growth with tellurite back to wild-type levels and to reestablish the uptake activity (Table 1). These complementation experiments showed that sensitivity and tellurite uptake were related. The construction of the plasmids used in this work is described in the supplemental material.
Cloning of the acetyl-CoA synthetase-acetate permease (acs-actP) operon.
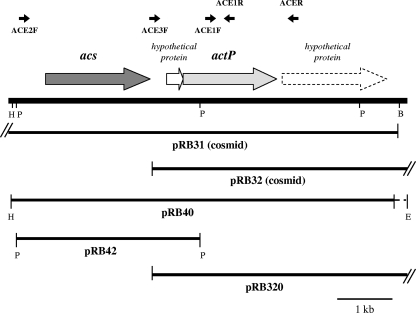
To identify the gene(s) present on the isolated cosmids and directly involved in complementation of the mutant, a mini-library, made after partial digestion of pRB32, was constructed in the broad-host-range vector pRKK13 and transferred by conjugation into RTE36. Plasmid pRB320 (Fig. 2), carrying a 7.5-kb DNA fragment, conferred upon the mutant a phenotype highly similar to that of the parent pRB32 (Table 1). A 0.8-kb EcoRI-PstI fragment and a 2.4-kb PstI fragment were cloned from pRB320 to give plasmids pRB321 and pRB322 and sequenced. A BLAST search made with both sequences led to the identification of the R. capsulatus acetate permease (actP) gene, which is present on a large contig of the partially sequenced R. capsulatus SB1003 genome (accession no. AF010496) (Fig. 2). The two fragments proved to be contiguous and comprise the entire actP gene. The EcoRI site on the 0.8-kb fragment does not correspond to any site on the R. capsulatus sequence, indicating that this fragment is likely positioned at the end of the sequence cloned in pRB320 (Fig. 2) and that the EcoRI site is either part of the multiple-cloning site (MCS) in pRKK13 or derives from the digested pRB32. In the sequence present in the data bank, actP is preceded by a small hypothetical protein and by the gene (acs) coding for acetyl coenzyme A (CoA) synthetase, which represents the same gene organization described in E. coli (6). A series of primers (see the supplemental material) were designed, based on the available sequence, in order to amplify different portions of the acs-actP operon. The amplification profiles, obtained using pRB31, pRB32, pRB320, and B100 genomic DNA as the templates (not shown), allowed the definition of the portion of the sequence covered by the two cosmids (Fig. 2). While all of the forward and reverse primers were positive in the amplification reactions using pRB31 or B100 genomic DNA, only ACE1F, ACE1R, and ACER (Fig. 2) gave PCR products when pRB32 and pRB320 were the templates (not shown). Thus, the fact that the primers ACE2F and ACE3F, both upstream of actP, do not recognize sequences on pRB32 and pRB320 and the position of the 0.8-kb EcoRI-PstI fragment, derived from pRB320, at the beginning of actP indicate that the R. capsulatus DNA cloned in pRB32 and pRB320 starts in the acs-actP intergenic region. Both plasmids, when conjugated into RTE36, restored TeO32− uptake (Table 1). These findings strongly suggest that actP, with the possible contribution of the small hypothetical protein upstream of it, is sufficient to complement the tellurite uptake defect present in RTE36 (Table 1). In order to substantiate this conclusion, two additional plasmids were constructed: pRB40, carrying both acs and actP, and pRB42, carrying acs only (Fig. 2). These new constructs were introduced by conjugation into the mutant RTE36 and tested for growth in the presence of tellurite and for uptake. pRB40 complemented the mutant for both absence of growth, with tellurite, and uptake, whereas pRB42 showed a phenotype identical to the “noncomplemented” mutant RTE36 (Table 1). The cloning experiments demonstrated that acetate permease is required and is sufficient to restore, in the mutant RTE36, the wild-type activity for tellurite uptake and the low-level resistance to the oxyanion typical of R. capsulatus B100.
FIG. 2.
Restriction map and gene organization of the actP genomic region. The small arrows above the gene map indicate the position of the primers used in this work. The lines in the lower half of the figure show the portion of the actP genomic region cloned in the indicated plasmid. Restriction sites relevant to the construction of the plasmids are indicated by E (EcoRI), H (HindIII), and P (PstI).
In E. coli, the genes acs and actP and the small gene yjcH, positioned in between, are cotranscribed, forming a single operon (6). R. capsulatus maintains, for these genes, the same organization found in E. coli (Fig. 2), where it was shown that no additional promoter(s) is present in the intergenic region between acs and yjcH (6). In the present study, we found that in R. capsulatus, a DNA fragment carrying the gene coding for “hypothetical protein-ActP” alone, without acs, is able to fully complement the tellurite uptake defect in the mutant RTE36 (Table 1). Our data clearly indicate that another promoter could be present in the acs-hypothetical protein gene intergenic region right upstream of actP, in addition to the promoter(s) in front of acs. This possibility is currently under investigation.
Supplementary Material
Acknowledgments
This work was supported by the Ministero Istruzione Università e Ricerca (PRIN 2005) and University of Bologna local funding (RFO 2008).
Footnotes
Published ahead of print on 4 December 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Borghese, R. 1992. Ph.D. thesis. University of Missouri, Columbia, MO.
- 2.Borghese, R., D. Marchetti, and D. Zannoni. 2008. The highly toxic oxyanion tellurite (TeO32−) enters the phototrophic bacterium Rhodobacter capsulatus via an as yet uncharacterized monocarboxylate transport system. Arch. Microbiol. 189:93-100. [DOI] [PubMed] [Google Scholar]
- 3.Borsetti, F., A. Toninello, and D. Zannoni. 2003. Tellurite uptake by cells of the facultative phototroph Rhodobacter capsulatus is a ΔpH dependent process. FEBS Lett. 554:315-318. [DOI] [PubMed] [Google Scholar]
- 4.Borsetti, F., V. Tremaroli, F. Michelacci, R. Borghese, C. Winterstein, F. Daldal, and D. Zannoni. 2005. Tellurite effects on Rhodobacter capsulatus cell viability and superoxide dismutase activity under oxidative stress conditions. Res. Microbiol. 156:807-813. [DOI] [PubMed] [Google Scholar]
- 5.Chasteen, T. G., D. E. Fuentes, J. C. Tantaleán, and C. C. Vásquez. 2009. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol. Rev. 33:820-832. [DOI] [PubMed] [Google Scholar]
- 6.Gimenez, R., M. F. Nuñez, J. Badia, J. Aguilar, and L. Baldoma. 2003. The gene yjcG, cotranscribed with the gene acs, encodes an acetate permease in Escherichia coli. J. Bacteriol. 185:6448-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomás, J. M., and W. W. Kay. 1986. Tellurite susceptibility and non-plasmid-mediated resistance in Escherichia coli. Antimicrob. Agents Chemother. 30:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner, M. S., Y. P. Tan, and P. M. Giffard. 2007. Inactivation of an iron transporter in Lactococcus lactis results in resistance to tellurite and oxidative stress. Appl. Environ. Microbiol. 73:6144-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver, P. F., J. D. Wall, and H. Gest. 1975. Characterization of Rhodopseudomonas capsulata. Arch. Microbiol. 105:207-216. [DOI] [PubMed] [Google Scholar]
- 10.Zannoni, D., F. Borsetti, J. J. Harrison, and R. J. Turner. 2008. The bacterial response to the chalcogen metalloids Se and Te. Adv. Microb. Physiol. 53:1-71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.