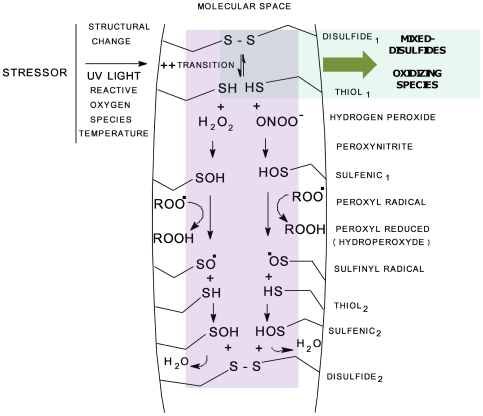
Figure 12. Theoretical model to explain the effect of a stressor on the changes in AC and RS of Human Serum Albumin (HSA).
In some places on the molecular space, stressors induce a transition state between disulfide (DISULFIDE1) and thiol groups (THIOL1). Reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO-) can react with thiols to form a sulfenic acid derivative (HSA-SOH) (SULFENIC1). The Sulfenic group is an efficient reducer of peroxy radicals (ROO) and reacts with it to form a sulfinyl radical derivative (HSA-SO) and the correspondent hydroperoxyde (ROOH). A new sulfenic acid derivative (SULFENIC2), this time formed by the reaction of previously formed HSA-SO. with new thiol groups (THIOL2), completes the cycle with the formation of additional disulfides (DISULFIDE2). Protein requires space to move itself between transition states (flexibility). The precise localization of cysteine residues facilitates the cycle. The driven force to move the entire system depicted should be the more favorable thermodynamic molecular state derived from the primary structure. Pale green = passive component; pale violet = active component or structural change dependent. Intersection is common to both components.