Abstract
Background: Docetaxel (T; Taxotere) with capecitabine (X) is active against metastatic breast cancer (MBC); bevacizumab (BV) has demonstrated efficacy with taxanes in the first-line setting. This study was conducted to assess the safety and efficacy of TX-BV in patients with MBC.
Patients and methods: In this single-arm, multicenter phase II study, patients received first-line bevacizumab 15 mg/kg and docetaxel 75 mg/m2 on day 1 and capecitabine 825 mg/m2 twice per day on days 1–14 every 21 days. Primary and secondary end points were tumor response rate (RR), overall survival (OS), progression-free survival (PFS), and toxicity.
Results: A total of 45 assessable patients received TX-BV for a median of seven cycles. Two complete and 20 partial responses were observed (overall RR 49%); nine patients had stable disease >6 months, for a clinical benefit rate of 69%. Median response duration was 11.8 months. Median OS and PFS were 28.4 and 11.1 months, respectively. Grade 3/4 adverse events included hand–foot syndrome (29%), fatigue (20%), febrile neutropenia (18%), and diarrhea (18%). In cycles 3–10, median dose levels of docetaxel and capecitabine were 60 mg/m2 and 660 mg/m2, respectively.
Conclusion: TX-BV demonstrated significant activity; dose modifications were required to manage drug-related toxic effects.
Keywords: bevacizumab, capecitabine, chemotherapy, docetaxel
introduction
Despite advances with hormonal and chemotherapeutic agents, the mean duration of survival from the time of diagnosis of advanced or metastatic breast cancer (MBC) ranges from 2 to 4 years [1], and the 5-year survival rate is 26.7% [2]. Chemotherapy is recommended as first-line treatment of patients with hormone receptor-negative tumors and for those whose tumor burden necessitates a rapid objective response [3]. For patients with MBC who have received anthracyclines within the course of adjuvant or neoadjuvant therapy, combination treatment with capecitabine and docetaxel (Taxotere) represents the current regulatory standard of care [4, 5].
Angiogenesis plays a critical role in the development and progression of many solid tumors [6]. Vascular endothelial growth factor (VEGF) is the most potent driver of normal and pathologic angiogenesis, is involved in the migration and mitogenesis of endothelial cells, induction of extracellular matrix remodeling, increased vascular permeability, and maintenance of newly formed blood vessels [7–9], and is also a negative prognostic indicator for breast cancer relapse and survival [10, 11]. Inhibition of VEGF signaling is an important strategy in the treatment of several malignancies, including breast cancer [12–17].
Bevacizumab is a recombinant, humanized, anti-VEGF mAb approved in combination with paclitaxel (Taxol) for the first-line treatment of human epidermal receptor 2 (HER2)-negative MBC [17]. The addition of bevacizumab to paclitaxel was shown to significantly increase the response rate (RR), median progression-free survival (PFS), and 1-year survival compared with paclitaxel alone in patients with previously untreated metastatic disease. More recently, a second phase III trial confirmed the benefit of bevacizumab plus docetaxel in the first-line treatment of MBC [18]. Given the antitumor activity achieved with the combined use of taxane therapy and bevacizumab, the addition of a VEGF inhibitor to the regimen of docetaxel and capecitabine is reasonable for clinical testing. This phase II study was designed to evaluate the efficacy and safety of first-line therapy with docetaxel, capecitabine, and bevacizumab in patients with MBC.
patients and methods
patients
Study enrollment was limited to men and women ≥18 years with cytologic or histologic confirmation of invasive breast cancer and clinical evidence of metastatic disease. Patients were eligible if they had at least one measurable lesion ≥2.0 cm in diameter by computed tomography (CT)/magnetic resonance imaging or ≥1.0 cm by spiral CT (superficial, clinically measurable lesions and clearly defined lesions on chest X-ray were also acceptable), no stage III or IV invasive nonbreast malignancies for ≥5 years, normal hematologic and general laboratory values, urinalysis ≤1+ protein, life expectancy of ≥3 months, and Eastern Cooperative Oncology Group performance status (ECOG PS) of zero or one. Patients with prior adjuvant or neoadjuvant treatment that was not for metastatic disease were permitted. Patients with HER2-positive tumors by immunohistochemistry or amplified FISH were excluded unless their disease had progressed after trastuzumab-containing therapy or trastuzumab was contraindicated.
Written informed consent was required. The protocol was approved by the institutional review board of each investigational site and was conducted in accordance with the Declaration of Helsinki, current USA Food and Drug Administration Good Clinical Practice guidelines, and institutional ethical and legal requirements.
study design and treatment
The North Central Cancer Treatment Group (NCCTG) study N0432 was a prospective, multicenter, single-stage phase II trial of first-line combination therapy with docetaxel, capecitabine, and bevacizumab in patients with MBC. Patients received treatment on a 3-week cycle, with dexamethasone 8 mg administered orally twice per day—the first dose given 12 h before starting chemotherapy, the second on the morning of chemotherapy, and the third on the evening of chemotherapy. Docetaxel 75 mg/m2 was given as a 250 ml i.v. infusion, using 5% dextrose as a diluent, over 1 h on day 1; capecitabine 825 mg/m2 was given orally twice per day on days 1 through 14; and bevacizumab 15 mg/kg was given i.v. on day 1. In patients with moderate renal impairment at baseline, the dose of capecitabine was reduced to 660 mg/m2. The initial bevacizumab dose was given over 90 min; if well tolerated, the second infusion was delivered over 60 min. Subsequent bevacizumab infusions were given over 30 min. Cycles were repeated every 3 weeks in the absence of disease progression or unacceptable toxicity. Patients received at least two additional cycles of therapy after the assessment of complete response (CR, Disappearance of all target lesions).
Dose reductions for adverse events were permitted. The doses of docetaxel and capecitabine were reduced to 60 mg/m2 and 660 mg/m2, respectively, in patients with related grade 2 or 3 adverse events after resolution to grade 1 or less. In the event of a third occurrence of grade 2 toxicity, a second occurrence of grade 3 toxicity, or a first occurrence of grade 4 toxicity, doses were reduced to 45 mg/m2 and 500 mg/m2, respectively. No dose reductions of bevacizumab were allowed.
assessments
Before registration and during the study, patients underwent complete medical examinations, including complete history and physical; measurement of ECOG PS; measurement of blood pressure, indicator lesions, and complete blood count; routine serum chemistry, including levels of total bilirubin, liver transaminases, alkaline phosphatase, and creatinine; urinalysis; and chest X-ray or CT. The primary end point was confirmed tumor RR by RECIST; responses were confirmed on two separate occasions at least 6 weeks apart. Secondary end points were PFS, overall survival (OS), duration of response, and safety. PFS was defined as the time from registration to documentation of disease progression. Disease progression was assumed in patients who died before documentation of progression, and patients who started treatment but were lost to follow-up were censored on the last evaluation day. OS was defined as the time from registration to death by any cause. Causes of death were classified as malignant disease, toxicity, other causes, and unknown. Duration of response was defined as the time from initial documentation of objective response to the first date of documented progression. Adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
statistical analysis
A minimum of 45 assessable patients were required to test the null hypothesis that tumor RR [defined as CR + partial response (PR) per RECIST divided by the number of assessable patients] was ≤40% (combination considered ineffective) against the alternative hypothesis of RR of ≥60% for 6 weeks (combination warrants further testing). The design yielded a 91% probability of detecting a true response of ≥60% at a 0.09 level of significance. Distributions of PFS and OS were estimated using the Kaplan–Meier method.
results
enrollment and baseline characteristics
Forty-six patients were recruited from 20 sites from December 2004 to September 2005. One patient, in whom treatment was never initiated, was removed from the analysis. Baseline characteristics of the 45 assessable patients are in Appendix A. Thirty-three patients (73%) had received systemic therapy in the adjuvant setting, including 25 (56%) with exposure to anthracyclines. HER2 status was negative in all patients with available data.
treatment
A median of seven treatment cycles (range 1 to 37 cycles) was given. Fewer than 50% of patients received the prescribed starting doses of docetaxel and capecitabine after cycle 2 (Appendix B). There were 45 docetaxel dose reductions in 30 patients. Reasons for docetaxel dose reductions included hematologic toxicity (38%); neurologic toxicity (20%); mucositis, stomatitis, and/or pharyngitis (11%); diarrhea (4%); skin toxicity (4%); infection (4%); and other (13%). Other reasons for docetaxel dose reduction were grade 3 nail changes (one patient); multiple toxic effects, including grade 3 fatigue, along with nail bed changes and watery eyes (one patient); grade 3 hand–foot syndrome and neuropathy (one patient); and hand–foot syndrome and primary investigator discretion (one patient). There were 75 capecitabine dose reductions in 35 patients. Reasons for capecitabine dose reductions were hand–foot skin reactions (48%); hematologic toxicity (13%); mucositis, stomatitis, and/or pharyngitis (12%); diarrhea (11%); infection (4%); and other (11%). Other reasons for capecitabine dose reductions were primary investigator discretion (three patients), hospitalization (two patients), hypertension (one patient), and incorrect dosage (one patient).
Next-cycle treatment delays occurred 35 times in 21 patients. Reasons for delay included hand–foot skin reactions (14%); infection (11%); elevated liver enzyme levels (9%); diarrhea (6%); neurologic toxicity (6%); mucositis, stomatitis, and/or pharyngitis (3%); skin toxicity (3%); hematologic toxicity (3%); and other (46%). Other causes for treatment delays were personal social reasons in four patients; chemotherapy holiday in two patients; and grade 2 proteinuria, disease progression, drug inventory issues, late-arriving capecitabine dosage, gastric biopsy and colonoscopy, port placement, therapy discontinuation, noncompliance with instructions, and surgical intervention in one patient each. All 45 patients have discontinued study treatment. Reasons for treatment discontinuation included disease progression (36%), adverse events (22%), patient refusal (18%), alternate treatment (13%), and other (11%); Other reasons were infection and open wound at breast implant incision site, death on study (disease progression), complete radiologic remission with grade 3 hand–foot syndrome, maximum benefit after eight cycles, and maximum response and expected mastectomy in one patient each.
response, survival, and disease progression
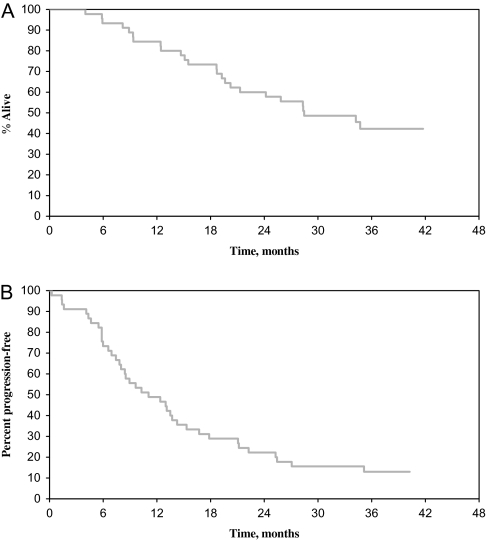
Tumor response data are available for all 45 assessable patients. Twenty-two patients [49%; 95% confidence interval (CI) 34% to 64%] had confirmed PR (n = 20) or CR (n = 2). The median duration of response was 11.8 months (range 2.4–38.9 months). Of the 22 responders, 18 subsequently developed tumors; nine patients had stable disease for >6 months for a clinical benefit responding 69%. Nineteen of 31 (61%) patients with estrogen receptor (ER)-positive disease responded (one CR and 18 PR), and 3 of 13 (23%) patients with ER-negative disease responded (one CR and two PR). The two patients with CR remained in disease remission for 10.7 and 24.5 months, respectively. Patients were followed until death or for a median of 36.2 months (range 27.9 to 41.8 months) for those patients who lived. The median OS was 28.4 months (95% CI 20.2 to not reached), and the median PFS was 11.1 months (95% CI 8.0–14.3 months) (Figure 1). The 1-year OS and PFS rates were 84% (95% CI 74% to 96%) and 49% (95% CI 36% to 66%), respectively.
Figure 1.
Kaplan–Meier plots for progression-free survival (A) and overall survival (B).
adverse events
Overall, 44 of 45 patients (98%) experienced at least grade 3 adverse events, and 31 of 45 patients (69%) experienced at least grade 4 adverse events (Table 1). Neutropenia was the most common grade 3 or 4 adverse event and was reported in 35 patients (78%). Other common severe adverse events included hand–foot syndrome (29%), fatigue (20%), febrile neutropenia (18%), diarrhea (no colostomy; 18%), nausea (13%), stomatitis (11%), and pharyngitis (9%). Growth factor support was required in 17 (38%) patients. Toxic effects specific to bevacizumab, such as hemorrhage and hypertension, occurred in a minority of patients and were primarily grade 1 or 2. One patient (2%) suffered grade 3 lower gastrointestinal bleed, two patients (4%) had grade 3 hypertension, and one patient (2%) had grade 4 thrombosis. Grade 3 neuropathy was reported in two patients (one case each of sensory and motor neuropathy).
Table 1.
Severe Adverse events experienced in at least 9% of patients
| Adverse event | Grade |
|
| 3 |
4 |
|
| Number of patients (%) | Number of patients (%) | |
| Neutropenia | 6 (13) | 29 (64) |
| Leukopenia | 22 (49) | 9 (20) |
| Skin reaction—hand–foot | 13 (29) | 0 (0) |
| Fatigue | 7 (16) | 2 (4) |
| Febrile neutropenia | 6 (13) | 2 (4) |
| Diarrhea—no colostomy | 7 (16) | 1 (2) |
| Nausea | 5 (11) | 1 (2) |
| Stomatitis | 4 (9) | 1 (2) |
| Vomiting | 3 (7) | 1 (2) |
| Pharyngitis | 4 (9) | 0 (0) |
discussion
The NCCTG N0432 study evaluated the efficacy and safety of first-line docetaxel, capecitabine, and bevacizumab in patients with MBC. Seventy-three percent of patients had received systemic therapy in the adjuvant setting, and 58% had had exposure to anthracycline-containing therapy. The combination regimen produced an RR of 49%, with median OS and PFS of 28.4 and 11.1 months, respectively. This level of activity exceeded the predefined threshold for an ineffective combination therapy (i.e. 40% RR). The clinical benefit rate (responses plus stable disease >6 months) was 69%. The regimen was moderately well tolerated, although myelosuppression was notable. The occurrence of adverse events necessitated growth factor support in 38% of patients as well as dose reductions of docetaxel and/or capecitabine in more than two-thirds of the patients in the study. There was, however, no apparent increase in bevacizumab-related adverse events and no augmentation of docetaxel- or capecitabine-related adverse events with the addition of bevacizumab.
Regimens consisting of capecitabine and a taxane have shown significant antitumor activity in phase II and III trials [4, 19–22]. In a phase III study of women with anthracycline-refractory MBC, the addition of capecitabine to docetaxel significantly improved the overall RR (42% versus 30%; P = 0.006), median time to progression (6.1 versus 4.2 months; P = 0.0001), and median OS (14.5 versus 11.5 months; P = 0.0126) compared with single-agent docetaxel [4]. Despite the associated toxic effects, the relative benefit to risk of docetaxel plus capecitabine was considered appropriate for approval in patients with anthracycline-pretreated disease.
The ongoing phase III study AVADO is evaluating first-line docetaxel 100 mg/m2 with placebo or bevacizumab (7.5 mg/kg or 15 mg/kg) every 3 weeks in patients with HER2-negative, locally recurrent breast cancer or MBC [18]. In the unstratified interim analysis, the addition of bevacizumab 7.5 or 15 mg/kg to docetaxel significantly increased the RR (55% and 63%, respectively, versus 44%; P ≤ 0.030) and median PFS (8.7 and 8.8 months, respectively, versus 8.0 months; P ≤ 0.032) compared with docetaxel plus placebo. The rate of febrile neutropenia was 12.0%–16.6% across the study arms.
Capecitabine has also been evaluated with bevacizumab in the treatment of patients with advanced breast cancer [23–25]. Interim data from a phase II study of first-line capecitabine 1000 mg/m2 twice per day plus bevacizumab demonstrated an RR of 38%, a median PFS of 5.7 months (95% CI 4.9–8.4 months), and a median OS of 16.0+ months (95% CI 12.9 to not reached) in 106 assessable patients [24]. The RIBBON-1 Study provided similar results [25].
Alternate schedules and/or lower doses of docetaxel and capecitabine have been used to reduce treatment-related adverse events without compromising efficacy of the combination [20, 26]. Doses of capecitabine and docetaxel in the present study were initially on the basis of adjuvant treatment in US Oncology trial 01-062. The rate of grade 3 or 4 neutropenia (78%) was nevertheless higher than anticipated but within the rate (83%) previously reported in a phase II trial of neoadjuvant capecitabine and docetaxel in patients with stage II or III invasive breast cancer [27]. In total, hematologic toxicity accounted for 38% and 13% of the dose reductions of docetaxel and capecitabine, respectively. The incidence of other grade 3 or 4 chemotherapy-related adverse events was similar to that reported previously with docetaxel and capecitabine [4].
The use of bevacizumab in the combination regimen did not result in any unexpected safety signals, and adverse events specific to bevacizumab were mild. It is notable that grade 3 or 4 hypertension occurred in only 4.4% of patients.
In summary, the overall RR, clinical benefit rate, median PFS, and median OS observed with docetaxel, capecitabine, and bevacizumab in the present study demonstrate that the combination has promising antitumor activity as a frontline regimen. Although the docetaxel and capecitabine doses that were tested required reductions or discontinuation, lower starting doses of docetaxel and/or capecitabine may provide a reasonable option. These preliminary data indicate that, with appropriate provisions for the management of associated toxic effects, the combination merits further study in patients suitable for first-line doublet chemotherapy regimens in a phase III setting.
funding
National Institutes of Health (CA25224); Breast Cancer Research Foundation (90128047); Roche (XEL450); Genentech (AVF3128s).
Acknowledgments
The authors attest to the originality of their work, which contains final data from the N0432 study. Data from this study were previously presented at the San Antonio Breast Cancer Symposium, 14–17 December 2006, San Antonio, TX. We thank the patients, physicians, nurses, and data managers and trial coordinators who participate in this study.
Appendix 1:
Baseline characteristics (N = 45)
| Characteristic | Number of patients | % |
| Median age, years (range) | 52 (36–73) | |
| Race | ||
| White | 43 | 96 |
| Asian | 1 | 2 |
| Not reported | 1 | 2 |
| ECOG PS | ||
| 0 | 30 | 67 |
| 1 | 15 | 33 |
| Predominant site of disease | ||
| Soft tissue | 11 | 24 |
| Osseous | 5 | 11 |
| Visceral | 28 | 62 |
| Unknown | 1 | 2 |
| Number of metastatic sites | ||
| 3–5 | 7 | 16 |
| 6 | 13 | 29 |
| 7 | 13 | 29 |
| 8–9 | 11 | 24 |
| Unknown | 1 | 2 |
| Estrogen receptor | ||
| Positive | 31 | 69 |
| Negative | 13 | 29 |
| Unknown | 1 | 2 |
| Progesterone receptor | ||
| Positive | 23 | 51 |
| Negative | 20 | 44 |
| Unknown | 2 | 4 |
| Previous adjuvant systemic therapy | ||
| Yes | 33 | 73 |
| No | 11 | 24 |
| Unknown | 1 | 2 |
| Previous hormone therapy | ||
| Yes | 14 | 31 |
| No | 30 | 67 |
| Unknown | 1 | 2 |
| Prior anthracycline | ||
| Adjuvant | 25 | 56 |
| Neoadjuvant | 1 | 2 |
| None | 19 | 42 |
| Prior taxane | ||
| Adjuvant | 17 | 38 |
| Neoadjuvant | 1 | 1 (2) |
| None | 27 | 60 |
| HER2 method of detection | ||
| Immunohistochemistry | 27 | 60 |
| FISH | 15 | 33 |
| Not done | 3 | 7 |
| HER2 status | ||
| Negative | 33 | 73 |
| Not done | 4 | 9 |
| Missing | 8 | 18 |
ECOG PS, Eastern Cooperative Oncology Group performance status; HER2, human epidermal receptor 2.
Appendix 2:
Dosing during the first 10 cycles of treatment
| Cycle | Docetaxel |
Capecitabine |
Bevacizumab |
|||||||||
| N | Median total dose administered | Median dose level administered | Full dose received | N | Median total dose administered | Median dose level administered | Full dose received | N | Median total dose administered | Median dose level administered | Full dose received | |
| mg | mg/m2 | % | mg | mg/m2 | % | mg | mg/m2 | % | ||||
| 1 | 45 | 136 | 75 | 100 | 45 | 36 400 | 825 | 98 | 45 | 1100 | 15 | 100 |
| 2 | 40 | 126 | 75 | 58 | 40 | 33 650 | 825 | 60 | 41 | 1084 | 15 | 100 |
| 3 | 36 | 120 | 60 | 47 | 36 | 32 200 | 660 | 44 | 37 | 1050 | 15 | 100 |
| 4 | 32 | 120 | 60 | 47 | 28 | 28 950 | 660 | 25 | 33 | 1010 | 15 | 100 |
| 5 | 31 | 120 | 60 | 45 | 26 | 31 625 | 660 | 23 | 32 | 1005 | 15 | 100 |
| 6 | 27 | 115 | 60 | 44 | 22 | 31 625 | 660 | 23 | 29 | 1000 | 15 | 100 |
| 7 | 22 | 117 | 62 | 45 | 19 | 28 000 | 660 | 26 | 23 | 1074 | 15 | 96 |
| 8 | 18 | 115 | 62 | 44 | 16 | 28 000 | 660 | 19 | 22 | 1060 | 15 | 95 |
| 9 | 14 | 119 | 62 | 43 | 13 | 32 200 | 660 | 23 | 18 | 1084 | 15 | 94 |
| 10 | 13 | 117 | 60 | 38 | 12 | 30 100 | 660 | 25 | 18 | 1084 | 15 | 94 |
References
- 1.Cardoso F, Di LA, Lohrisch C, et al. Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades? Ann Oncol. 2002;13:197–207. doi: 10.1093/annonc/mdf101. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, et al. Cancer statistics 2009. J Clin Oncol. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Burzykowski T, Buyse M, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–1986. doi: 10.1200/JCO.2007.10.8399. [DOI] [PubMed] [Google Scholar]
- 4.O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20:2812–2823. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Clemons M. First-line treatment options for patients with HER-2 negative metastatic breast cancer: the impact of modern adjuvant chemotherapy. Oncologist. 2007;12:785–797. doi: 10.1634/theoncologist.12-7-785. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak HF, Nagy JA, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 9.Mercurio AM, Lipscomb EA, Bachelder RE. Non-angiogenic functions of VEGF in breast cancer. J Mammary Gland Biol Neoplasia. 2005;10:283–290. doi: 10.1007/s10911-006-9001-9. [DOI] [PubMed] [Google Scholar]
- 10.Gasparini G, Toi M, Miceli R, et al. Clinical relevance of vascular endothelial growth factor and thymidine phosphorylase in patients with node-positive breast cancer treated with either adjuvant chemotherapy or hormone therapy. Cancer J Sci Am. 1999;5:101–111. [PubMed] [Google Scholar]
- 11.Linderholm B, Tavelin B, Grankvist K, et al. Vascular endothelial growth factor is of high prognostic value in node-negative breast carcinoma. J Clin Oncol. 1998;16:3121–3128. doi: 10.1200/JCO.1998.16.9.3121. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 13.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 15.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 16.Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007;25:4536–4541. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 17.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 18.Miles D, Chan A, Romieu G, et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. J Clin Oncol. 2008;26(15s):1008s. (Abstr LBA1011) [Google Scholar]
- 19.Perez EA. Novel enhanced delivery taxanes: an update. Semin Oncol. 2007;34(3) Suppl:1–5. doi: 10.1053/s0093-7754(07)00088-7. [DOI] [PubMed] [Google Scholar]
- 20.Mrozek E, Ramaswamy B, Young D, et al. Phase II study of weekly docetaxel and capecitabine in patients with metastatic breast cancer. Clin Breast Cancer. 2006;7:141–145. doi: 10.3816/CBC.2006.n.023. [DOI] [PubMed] [Google Scholar]
- 21.Blum JL, Dees EC, Chacko A, et al. Phase II trial of capecitabine and weekly paclitaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2006;24:4384–4390. doi: 10.1200/JCO.2005.05.1383. [DOI] [PubMed] [Google Scholar]
- 22.Lueck H, von Minckwitz G, Du Bois A, et al. Epirubicin/paclitaxel (EP) vs. capecitabine/paclitaxel (XP) in first-line metastatic breast cancer (MBC): a prospective, randomized multicentre phase III study of the AGO breast cancer study group. J Clin Oncol. 2006;24(18s):7s. (Abstr 517) [Google Scholar]
- 23.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 24.Sledge G, Miller K, Moisa C, et al. Safety and efficacy of capecitabine (C) plus bevacizumab (B) as first-line in metastatic breast cancer. J Clin Oncol. 2007;25(18s):35s. (Abstr 1013) [Google Scholar]
- 25.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) J Clin Oncol. 2009;27(15s):42s. doi: 10.1200/JCO.2010.28.0982. (Abstr 1005) [DOI] [PubMed] [Google Scholar]
- 26.Leonard R, O'Shaughnessy J, Vukelja S, et al. Detailed analysis of a randomized phase III trial: can the tolerability of capecitabine plus docetaxel be improved without compromising its survival advantage? Ann Oncol. 2006;17:1379–1385. doi: 10.1093/annonc/mdl134. [DOI] [PubMed] [Google Scholar]
- 27.Lebowitz PF, Eng-Wong J, Swain SM, et al. A phase II trial of neoadjuvant docetaxel and capecitabine for locally advanced breast cancer. Clin Cancer Res. 2004;10:6764–6769. doi: 10.1158/1078-0432.CCR-04-0976. [DOI] [PubMed] [Google Scholar]