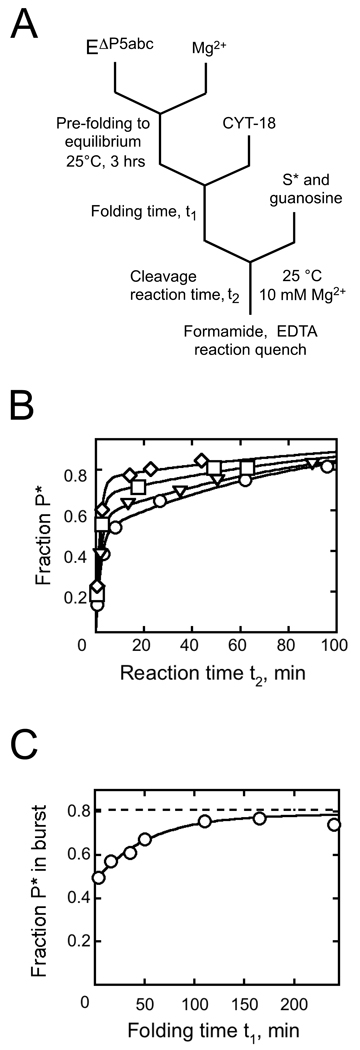
Fig. 2.
Native state stabilization by CYT-18 measured by ribozyme activity. A, Schematic of reaction procedure. B, Substrate cleavage reactions to give oligonucleotide product (P*) after incubation during t1 of 200 nM EΔP5abc with 500 nM CYT-18 for: 3.6 min (circles), 16 min (triangles), 110 min (squares), and 210 min (diamonds). C, The fraction of S* cleaved rapidly to P* (bursts from panel B) plotted against CYT-18 incubation time. Immediately after CYT-18 addition, the fraction of substrate cleaved was 0.48 ± 0.02. The fraction of misfolded ribozyme is inferred from the difference between this value and the maximum burst amplitude after extended incubations of the same ribozyme preparation with P5abc at elevated temperature (0.81 ± 0.02, dashed line; typically 30 min at 50 °C). The ratio of native to misfolded ribozyme gives an equilibrium constant of 1.5 ± 0.1 for folding of the EΔP5abc ribozyme, in good agreement with the value of 1.4 reported previously.22 At later times, the fraction of native ribozyme increased, with kobs = 0.019 ± 0.003 min−1, to a value indistinguishable from that obtained by incubation with P5abc, indicating that CYT-18 shifts the equilibrium to a value of at least 50 (see Results).