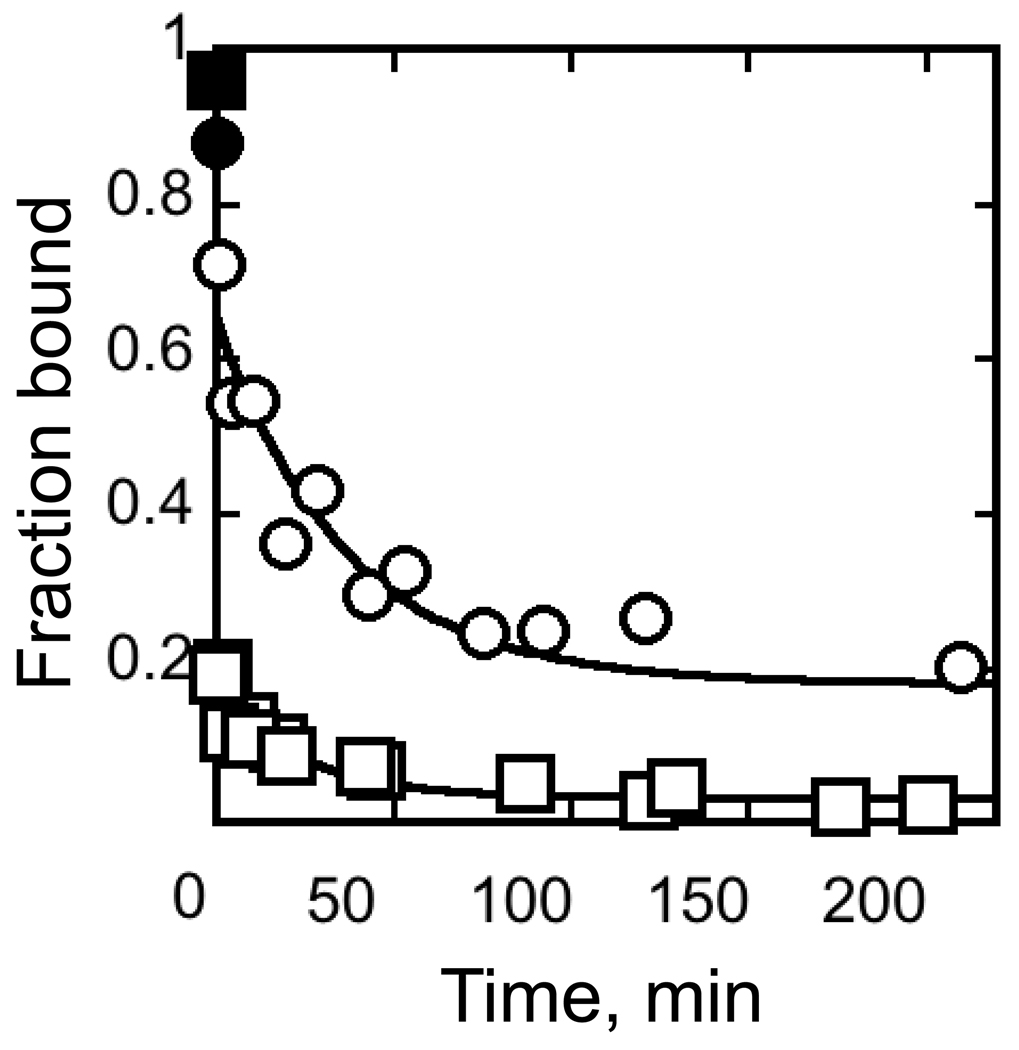
Fig. 4.
CYT-18 dissociation kinetics. CYT-18 (200 nM) was incubated with trace radiolabeled EΔP5abc ribozyme (~3 nM) to give populations of largely native or misfolded ribozyme (circles and squares, respectively; see Methods). Complex dissociation was then followed upon addition of excess unlabeled EΔP5abc (2 µM). Filled symbols show results without unlabeled ribozyme (again, circles and squares represent native and misfolded ribozyme, respectively). For the reaction with native ribozyme, the major phase gave a rate constant of 0.024 ± 0.018 min−1. The endpoint, 0.16 ± 0.05, is in the range expected from the 10-fold excess of unlabeled ribozyme, approximately 60% of which is expected to be native (with most of the remainder misfolded). In experiments monitoring dissociation from misfolded ribozyme, most of the labeled RNA dissociated before the first time point. A minor phase gave a rate constant of 0.042 ± 0.034 with an amplitude of ~0.1. The endpoint, 0.02 ± 0.018, is lower than in reactions with the native ribozyme, despite the same 10-fold excess of unlabeled ribozyme chase, presumably because the labeled misfolded ribozyme competes poorly with the native ribozyme in the chase.