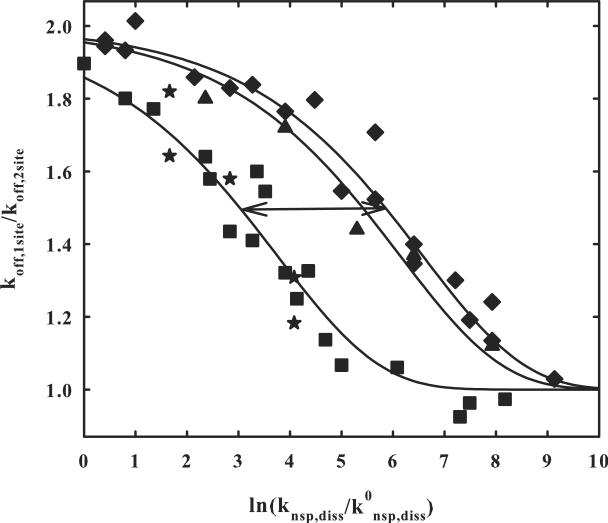
Figure 3.
The dependence of the ratio of the dissociation rates of EcoRI from DNA with one and two binding sites on changes in the nonspecific dissociation rate. The ratio of one- and two-site fragment dissociation rate constants determined in figure 2 is shown as a function of ln(knsp,diss/k0nsp,diss); k0nsp,diss is the nonspecific complex dissociation rate at 90 mM NaCl, 0 osmolal, pH 7.0, and 20 °C and knsp,diss is the nonspecific complex dissociation rate at the salt and osmolyte concentrations used. From our previous data, ln(knsp,diss/k0nsp,diss) is given by equation (7). The salt concentration is varied from 90 to 360 mM NaCl and the betaine glycine osmolyte concentration from 0 to 4 osmolal to give the range examined.
The ratios of dissociation rates for the fragment with two sites a separated by 84 bp is given by (■). The `star' (★) data points for the Δ84 bp two site fragment were obtained keeping the osmolal concentration of betaine glycine constant and only varying the salt concentration. Dissociation rate ratios for the Δ21 bp two site fragment are shown by (◆) and for the Δ26 two site fragment by (▲). The two sites are on the same side of the DNA helix for Δ84 and Δ21, but on opposite sides for Δ26. The solid lines are calculations of equation (6) with ln(k0nsp,diss/ksl) = −12.6. The arrow represents a 16-fold change in knsp,diss for the 4-fold change in spacing between sites as expected for a diffusion process. A complete data set that includes osmolal and salt concentrations, koff,1-site, and koff,2-site is given in supplementary materials for the Δ21 and Δ84 two site DNA fragments, as well as a graph of fitting errors.